Preparation method of sea squirt compound and intermediate thereof
A compound and transformation technology, applied in the field of medicine, can solve the problems of many side reactions, deprotection of phenolic hydroxyl groups, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
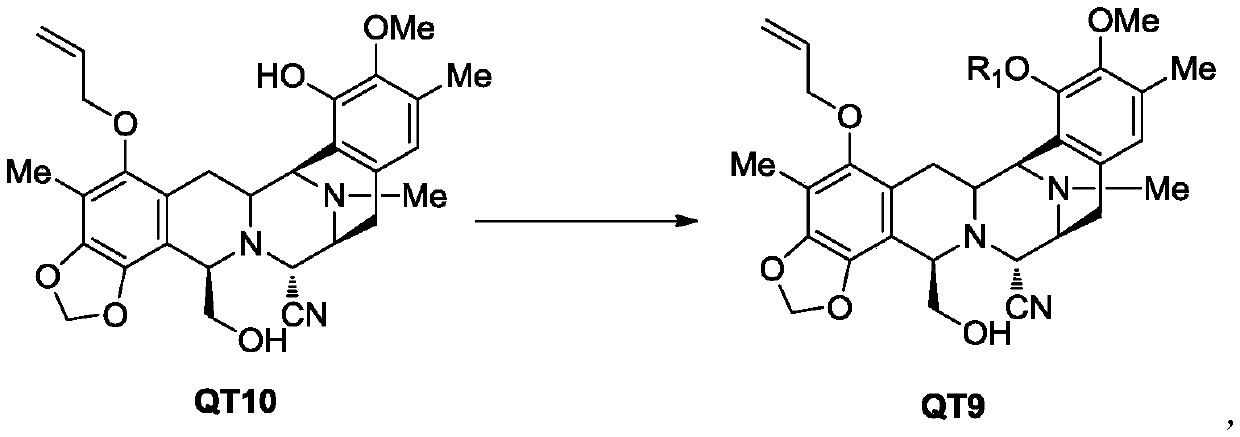
[0096] The synthesis of embodiment 1 compound 2
[0097]
[0098] 6.9 g of sodium hydroxide and 250 ml of tetrahydrofuran were added to a 1000 ml three-necked flask, the temperature was lowered to below 0°C, 59.8 g of compound QT10 was added, and 21.5 g of 2-methoxyethoxymethyl chloride (MEM-Cl) was added dropwise. After the dropwise addition, keep warm at 0-10°C and stir for 4 hours, quench with saturated ammonium chloride aqueous solution, extract with dichloromethane (2×500ml) and combine the organic layers, dry over anhydrous sodium sulfate, and concentrate in vacuo. Ethyl acetate was recrystallized from n-hexane to obtain 64.2 g of white solid, yield: 91.8%, HPLC>99%.
[0099] 1 HNMR (400MHZ, CDCl 3 ):δ6.71(s,1H),06.10(m,1H),5.93(d,J=1.2Hz,1H),5.87(d,J=1.2Hz,1H),5.44(dd,J1=1.2Hz ,J2=17.2Hz,1H),5.30(dd,J1=1.2Hz,J2=10.4Hz,1H),5.27(d,J=6Hz,1H),5.18(d,J=6Hz,1H),4.26( d,J=2.4Hz,1H),4,18-4.14(m,2H),4.04(d,J=2.4Hz,1H),3.99(t,2H),3.86(m,1H),3.66(s ,3H),3.64(m,1H),3.60(t,2...
Embodiment 2
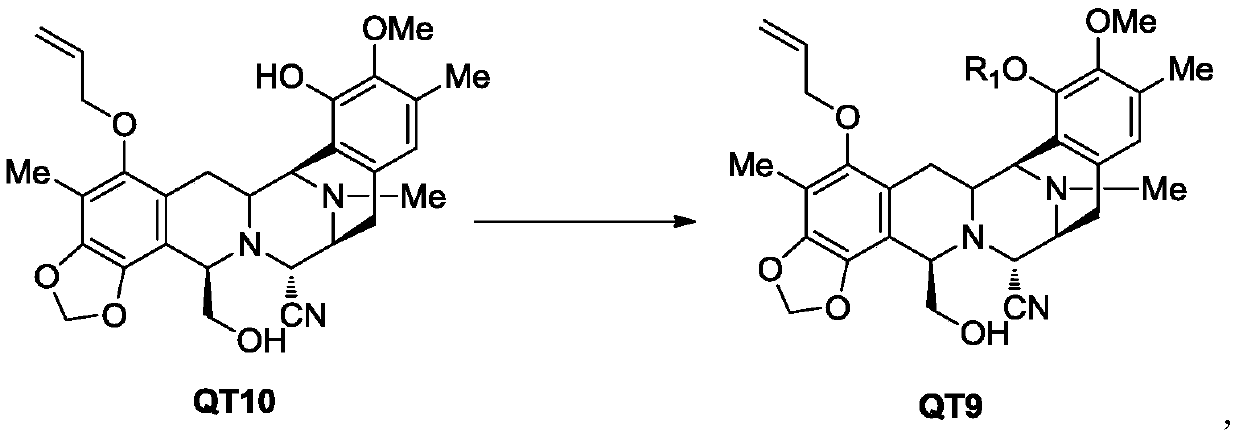
[0100] The synthesis of embodiment 2 compound 3
[0101]
[0102] Add 1.15 g of sodium hydroxide and 115 ml of tetrahydrofuran into a 500 ml three-necked flask, cool down to below 0° C., add 10.0 g of compound QT10, and dropwise add 2.41 g of bromomethyl methyl ether (MOM-Br). After the dropwise addition, keep warm at -5~5°C and stir for 2 hours, quench with saturated ammonium chloride aqueous solution, extract with dichloromethane (2×200ml), combine the organic layers, dry over anhydrous sodium sulfate, and concentrate in vacuo, the obtained foamy The solid was recrystallized from ethyl acetate and n-hexane to obtain 9.8 g of white solid, yield: 90.3%, HPLC>99%.
[0103] 1 HNMR (400MHZ, CDCl 3 ):δ6.72(s,1H),6.16-6.07(m,1H),5.93(d,J=1.6Hz,1H),5.88(d,J=1.6Hz,1H),5.44(dd,J1= 1.6Hz, J2=17.2Hz, 1H), 5.30(dq, J1=1.2Hz, J2=10.4Hz, 1H), 5.12(s, 2H), 4.27(d, J=2.0Hz, 1H), 4,12 -4.11(m,2H),4.05(d,J=2.4Hz,1H),3.99(t,J=3.2Hz,2H),3.71(s,3H),3.68-3.63(dt,J1=3.2Hz, J2=10.8Hz, 1H), 3...
Embodiment 3
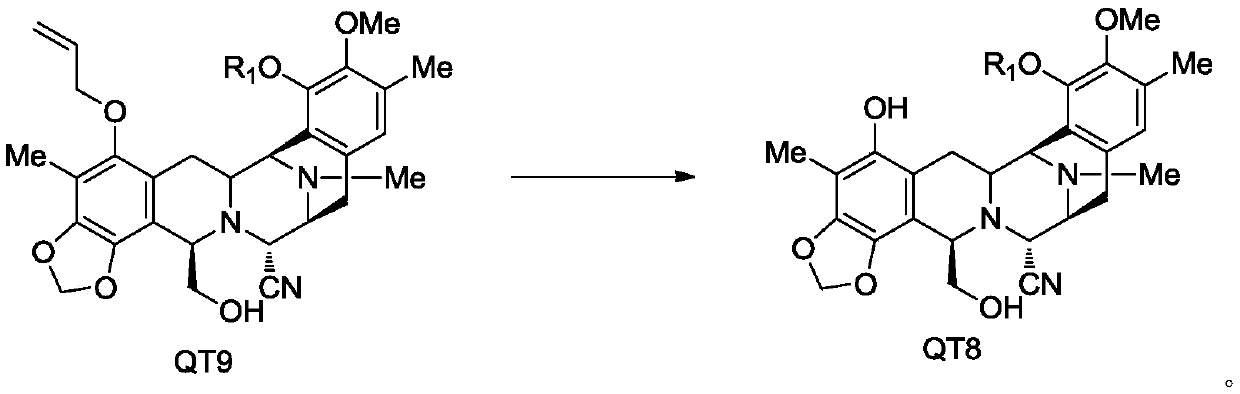
[0104] The synthesis of embodiment 3 compound 4
[0105]
[0106] Under the protection of argon, add 3.0 g of compound 2 to a 100 ml three-necked flask, add 0.241 g of bis(triphenylphosphine) palladium dichloride, 1.48 g of acetic acid and 60 ml of dichloromethane, and add 3.57 g of tri-n-N After the addition of butyltin hydrogen, keep warm at 0-5°C and stir for 1 hour, quench with saturated aqueous potassium fluoride, extract with dichloromethane (2×40ml), combine the organic layers, dry over anhydrous sodium sulfate, and concentrate in vacuo to obtain an oil. Column chromatography (n-hexane:ethyl acetate=4:1~1:1) gave 2.76 g of white foamy solid, yield: 98.5%, HPLC>98%.
[0107] 1 HNMR (400MHZ, CDCl 3 ):δ6.68(s,1H),5.89(d,J=1.2Hz,1H),5.82(d,J=1.2Hz,1H),5.44(dd,J1=1.2Hz,J2=17.2Hz,1H ), 5.30(dd, J1=1.2Hz, J2=10.4Hz, 1H), 5.61(s, 1H), 5.39(d, J=6Hz, 1H), 5.27(d, J=6Hz, 1H), 4.26( d,J=2.4Hz,1H),4,13-4.066(m,2H),3.99-3.93(m,2H),3.69(s,3H),3.68-3.65(m,3H),3.60(t, 1H), 3.40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com