Method for constructing 3,5-disubstituted pyridine by utilizing mixed styrene derivative and N,N-dimethylformamide
A technology of styrene derivatives and dimethylformamide, which is applied in the field of synthesis of mixed 3,5-disubstituted pyridine and 3,5-disubstituted pyridine, can solve the problems of low cost of raw materials and catalysts, mild reaction conditions, and the need for Metal catalysis and other problems, to achieve the effects of low cost, mild reaction conditions, and expanded production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
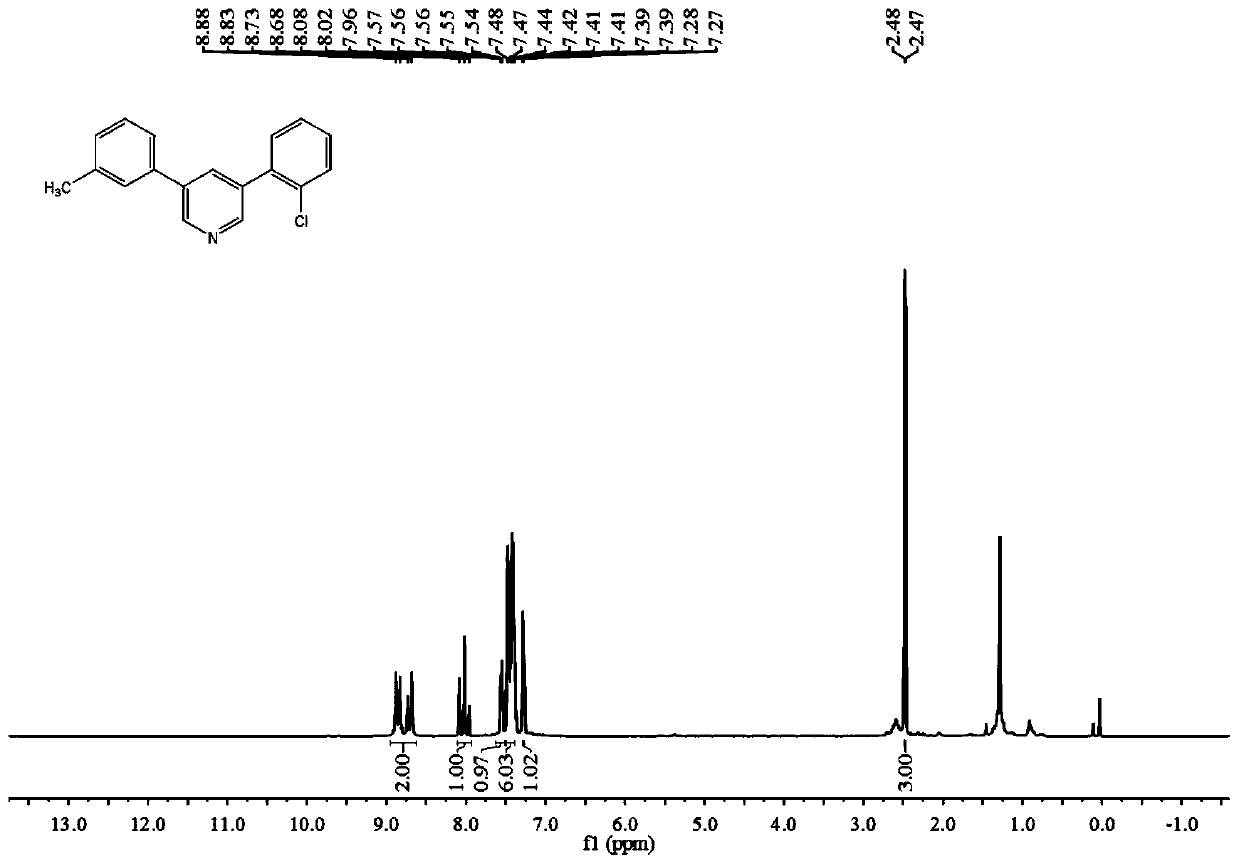
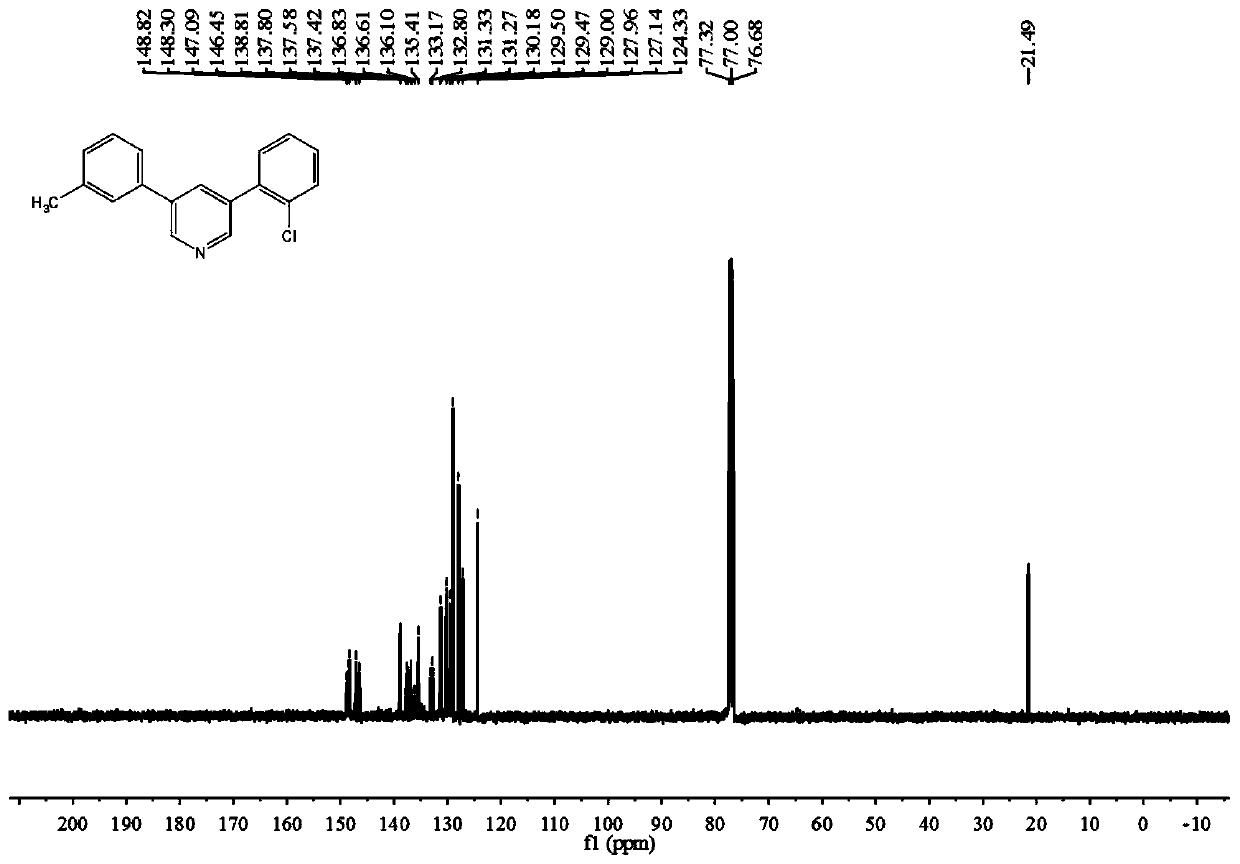
[0087] The Reaction of 3-Methylstyrene, 2-Chlorostyrene and DMF
[0088]
[0089] The specific operation process of the experiment: add 26mg (0.25mmol) of styrene, 34.5mg (0.25mmol) of 2-chlorostyrene, 405mg (1.5mmol) of potassium peroxodisulfate (K 2 S 2 o 8 ), 124.5 mg (0.75 mmol) of potassium iodide (KI) and 2 mL of N,N-dimethylformamide (DMF). Seal the reaction in an oil bath at 140° C. for 24 hours and take out the sealed tube to stop the reaction. After the temperature dropped to room temperature, 10 mL of sodium chloride (NaCl) aqueous solution and 10 mL of ethyl acetate (EtOAc) were added to the mixture. ) to repeat the extraction 3 times. After removing the residual moisture in the organic phase with anhydrous sodium sulfate, spin dry the organic solvent in the organic phase with a rotary evaporator. The concentrated reaction mixture was separated and purified through a silica gel column to obtain a yellow solid product with a yield of 43%.
[0090] Character...
Embodiment 2
[0092] The Reaction of 3-Methylstyrene, 4-Chlorostyrene and DMF
[0093]
[0094] The specific operation process of the experiment: 26mg (0.25mmol) of styrene, 34.5mg (0.25mmol) of 3-chlorostyrene, 405mg (1.5mmol) of potassium peroxodisulfate (K 2 S 2 o 8 ), 124.5 mg (0.75 mmol) of potassium iodide (KI) and 2 mL of N,N-dimethylformamide (DMF). Seal the reaction in an oil bath at 140° C. for 24 hours and take out the sealed tube to stop the reaction. After the temperature dropped to room temperature, 10 mL of sodium chloride (NaCl) aqueous solution and 10 mL of ethyl acetate (EtOAc) were added to the mixture. ) to repeat the extraction 3 times. After removing the residual moisture in the organic phase with anhydrous sodium sulfate, spin dry the organic solvent in the organic phase with a rotary evaporator. The concentrated reaction mixture was separated and purified through a silica gel column to obtain a yellow solid product with a yield of 49%.
[0095] Characterizat...
Embodiment 3
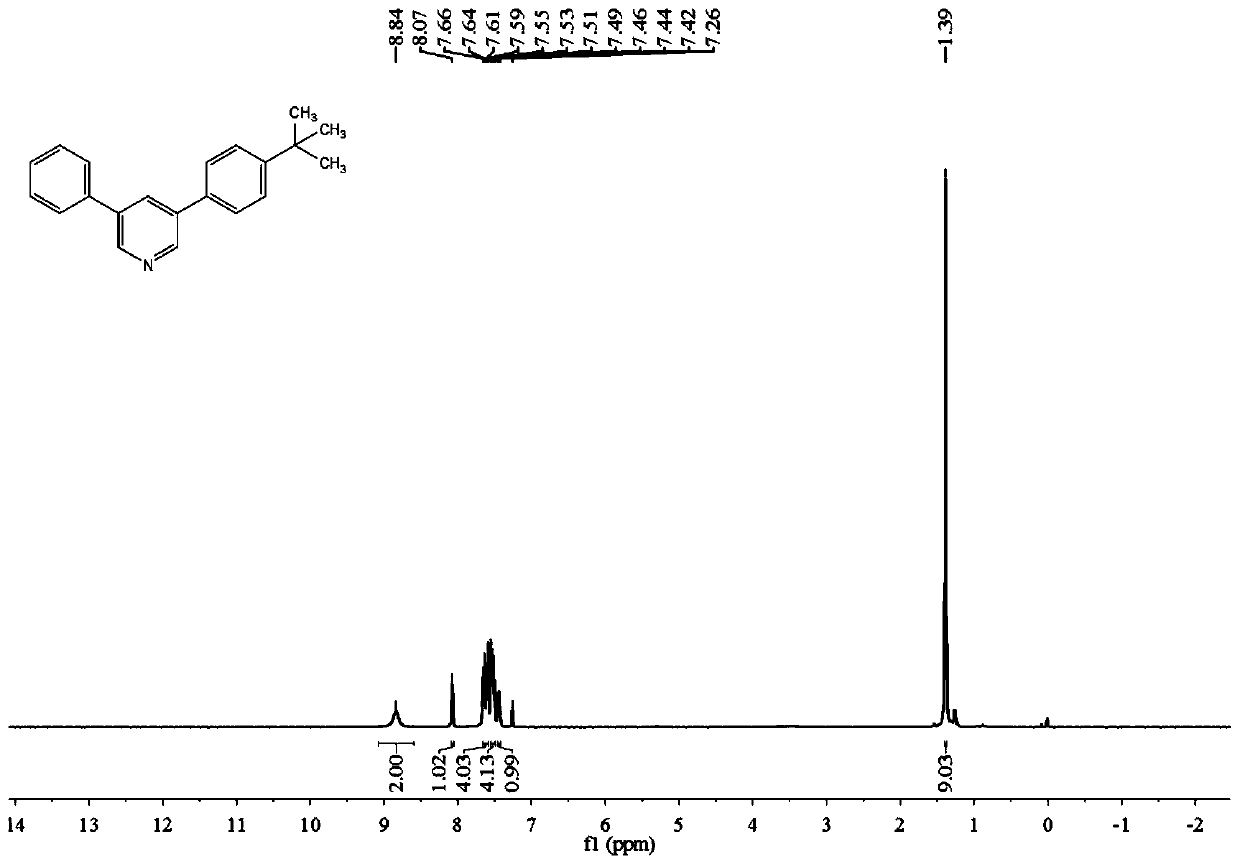
[0097] Reaction of Styrene, 4-tert-Butylstyrene and DMF
[0098]
[0099] The specific operation process of the experiment: add 26mg (0.25mmol) of styrene, 40mg (0.25mmol) of 4-tert-butylstyrene, 405mg (1.5mmol) of potassium peroxodisulfate (K 2 S 2 o 8 ), 124.5 mg (0.75 mmol) of potassium iodide (KI) and 2 mL of N,N-dimethylformamide (DMF). Seal the reaction in an oil bath at 140° C. for 24 hours and take out the sealed tube to stop the reaction. After the temperature dropped to room temperature, 10 mL of sodium chloride (NaCl) aqueous solution and 10 mL of ethyl acetate (EtOAc) were added to the mixture. ) to repeat the extraction 3 times. After removing the residual moisture in the organic phase with anhydrous sodium sulfate, spin dry the organic solvent in the organic phase with a rotary evaporator. The concentrated reaction mixture was separated and purified through a silica gel column to obtain a yellow solid product with a yield of 56%.
[0100] Characterizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com