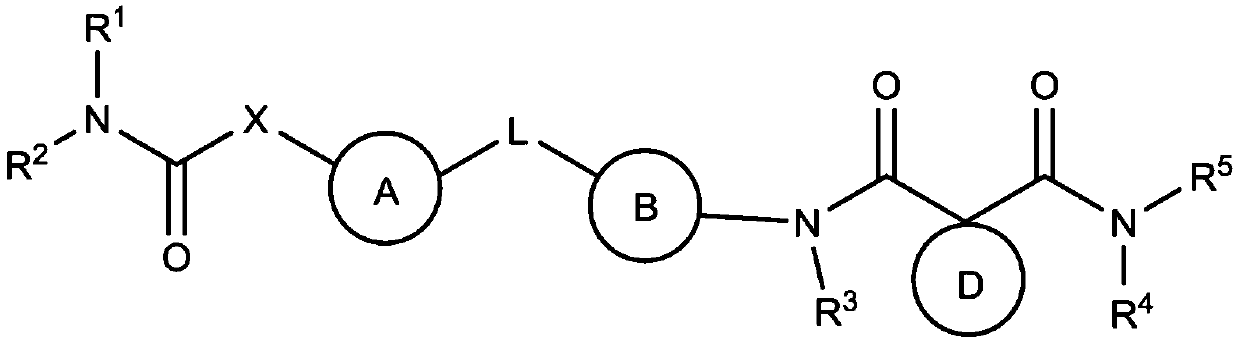
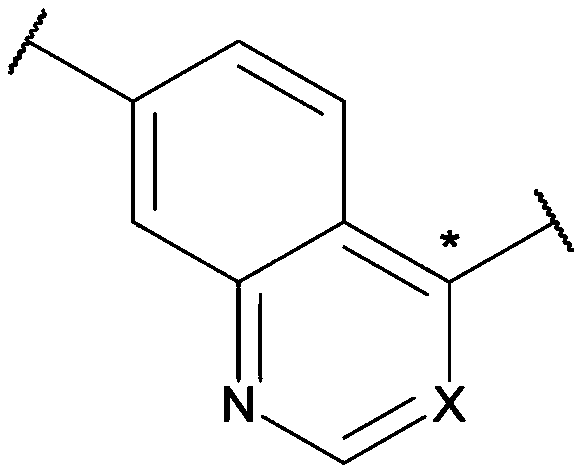
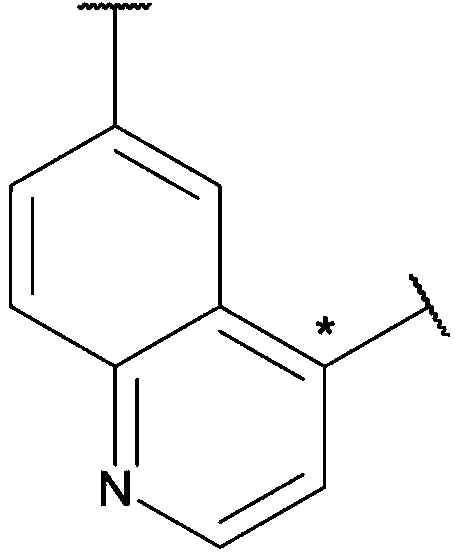
Carbamate and urea compounds as multikinase inhibitors
A compound and hydrocarbon-based technology, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve the problems of increased kinase-specific activity, overexpression or loss of negative regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0303] Example: Ex. or ex.
[0304] Formic acid: FA
[0305] Gram: g
[0306] High Performance Liquid Chromatography: HPLC
[0307] Inhibition: Inh.
[0308] Liquid Chromatography Mass Spectrometry: LCMS or LC-MS
[0309] Methanol: MeOH
[0310] Microliter: μL
[0311] Micrometer: μm
[0312] milligram: mg
[0313] Milliliter: mL
[0314] millimole: mmol
[0315] Nuclear magnetic resonance spectroscopy (Nuclear magnetic resonance spectroscopy): NMR
[0316] Phosphoryl chloride: POCl 3
[0317] Preparative HPLC: Prep-HPLC
[0318] Preparative TLC: Prep-TLC
[0319] Retention time: t R
[0320] Room temperature (ambient temperature, ~25°C): rt or RT
[0321] Potassium tert-butoxide: t-BuOK
[0322] Preparative HPLC: Prep-HPLC
[0323] Preparative TLC: Prep-TLC
[0324] Sodium methoxide: NaOMe
[0325] Supercritical Fluid Chromatography: SFC
[0326] temperature: temp.
[0327] Tetrahydrofuran (Tetrahydrofuran): THF
[0328] Thin layer chromatography: TLC ...
example 1
[0340] Example 1: 4-(4-(1-((4-fluorophenyl)carbamoyl)cyclopropane-1-carboxamido)phenoxy)-6-methoxy Quinolin-7-yl 4-methylpiperazine-1-carboxylate (4-(4-(1-((-fluorophenyl)carbamoyl)cyclopropane- 1-carboxamido)phenoxy)-6-methoxyquinolin-7-yl4-methylpiperazine-1- carboxylate) synthetic scheme 1
[0341]
[0342] step 1:
[0343] Add 12.5g (90.5mmol) K 2 CO 3 and 11.3g (66.07mmol) bromotoluene (benzyl bromide) to 10.0g (60.2mmol) 1-(4-hydroxyl-3-methoxyphenyl) ethane-1-ketone (1-( 4-hydroxy-3-methoxyphenyl)ethan-1-one) stirred solution. The reaction mixture was stirred at 40 °C under N2 atmosphere for 1 h. Diluted with 500 mL of water, and filtered; the filter cake was washed with water to obtain compound 1-1, which was used in the next step without further purification. LC-MS: m / e=257[M+H] + .
[0344] Step 2:
[0345] Add 10.6 mL (238.1 mmol) HNO dropwise at 0 °C 3 To a stirred solution of 26.0 g (101.6 mmol) of compound 1-1 in 400 mL of dichloromethane. Aft...
example 2
[0367] Example 2: 4-(4-(1-((-fluorophenyl)carbamoyl)cyclopropane-1-carboxamido)phenoxy)-6-methoxyquin Lin-7-yl (R)-3,4-methoxyquinoline 1-carboxylate (4-(4-(1-((-fluorophenyl)carbamoyl) cyclopropane-1-carboxamido)phenoxy)-6-methoxyquinolin-7-yl(R)-3,4- Synthetic Scheme 2 of dimethylpiperazine-1-carboxylate)
[0368]
[0369] step 1:
[0370] Add 0.40 g (3.1 mmol) DIEA and 0.46 g (2.3 mmol) 4-nitrophenyl carbonochloridate in 2 mL THF to 1.0 g ( 2.1 mmol) stirred solution of compound 1-9. The mixture was stirred at room temperature for 2 hours and then quenched with water. Extract with three 50 mL portions of ethyl acetate; the combined organic extracts were washed with brine and washed with anhydrous Na 2 SO 4 dry. After filtration, the filtrate was concentrated under vacuum to provide compound 2-1. LC-MS: m / e=653[M+H] + .
[0371] Step 2:
[0372] At room temperature, add 0.078g (0.77mmol) Et 3 N and 0.068g (0.46mmol) (R)-1,2-dimethylpiperazine hydrochlorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com