Method for improving conversion rate of chiral amine
A conversion rate and chiral amine technology, applied in the chemical field, can solve problems such as improving the conversion rate of chiral amines, and achieve the effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A method for improving the conversion rate of chiral amines, comprising the steps of:
[0049] (1) In vitro synthesis and amplification of genes:
[0050] Obtain the amino acid sequence of the (R)-selective type omega transaminase derived from Arthrobacter from the NCBI database, and the (R)-selective type omega transaminase is abbreviated as: ArR-ωTA; the amino acid sequence of the ArR-ωTA is as SEQ ID NO .1 shown;
[0051] The amino acid sequence of the ArR-ωTA is on the JCAT website, and the optimized ArR-ωTA nucleotide sequence is obtained by inputting: avoiding XbaI, NdeI, SpeI and HindIII restriction sites as conditions;
[0052] Before and after the optimized ArR-ωTA nucleotide sequence, XbaI restriction enzyme cutting site and HindIII restriction enzyme cutting site were respectively added to obtain the optimized ArR-ωTA nucleotide sequence containing restriction enzyme cutting site, The optimized ArR-ωTA nucleotide sequence containing restriction sites is shown...
Embodiment 2
[0066] A method for improving the conversion rate of chiral amines, comprising the steps of:
[0067] (1) with embodiment 1 step (1);
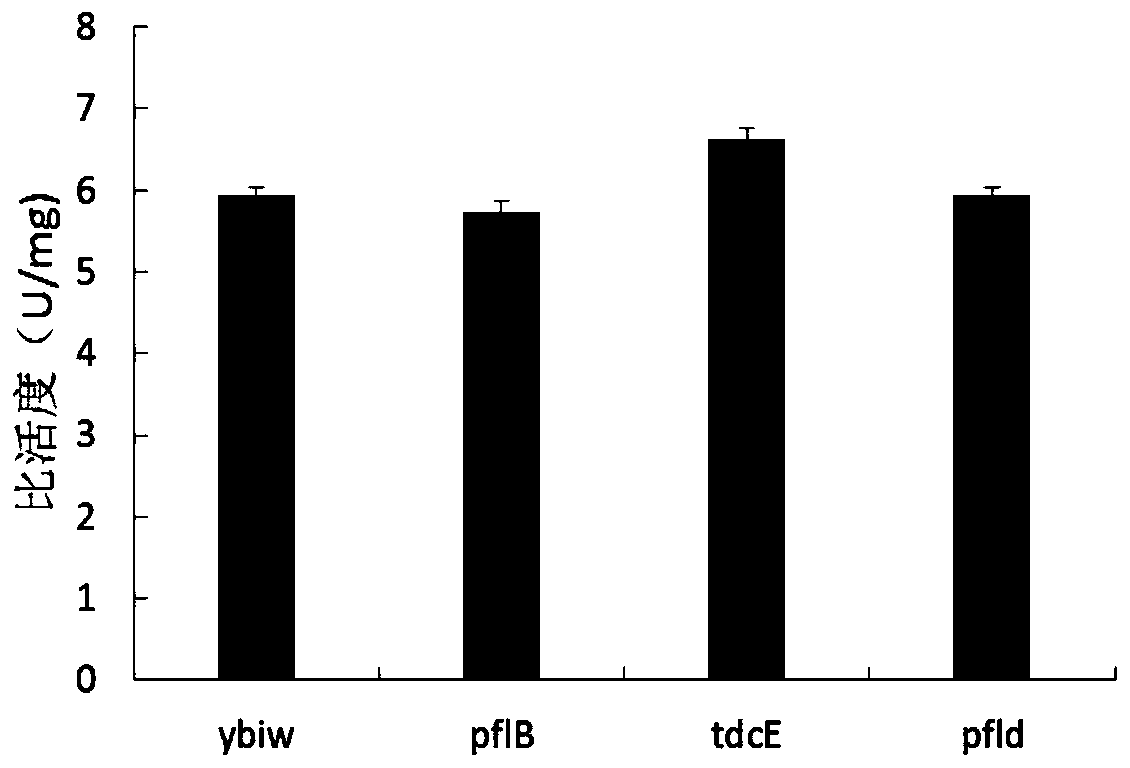
[0068] (2) replace tdcE in Example 1 with ybiw, and others are the same as Example 1 step (2);
[0069] (3) Introduce p2A4-ArR-ωTA; p2A4-ybiw; p2A4-ldh into Escherichia coli (E.coli MG1655) respectively to obtain recombinant bacteria 1, recombinant bacteria 2 and recombinant bacteria 6;
[0070] (4) Fermenting recombinant bacteria 1, recombinant bacteria 2 and recombinant bacteria 6 respectively to obtain recombinant bacteria 1 fermentation broth, recombinant bacteria 2 fermentation broth and recombinant bacteria 6 fermentation broth;
[0071] Fermentation process is with embodiment 1;
[0072] (5) The fermentation broth obtained in step (4) was centrifuged at 4°C and 6500rpm to collect the precipitate, and the precipitate was washed with 0.01M PBS (pH=7.5) to obtain the recombinant bacteria 1 fermentation broth precipitation, recombinant ba...
Embodiment 3
[0076] A method for improving the conversion rate of chiral amines, comprising the steps of:
[0077] (1) with embodiment 1 step (1);
[0078] (2) Replace tdcE in Example 1 with pflB, and others are the same as Example 1 step (2);
[0079] (3) Introduce p2A4-ArR-ωTA; p2A4-pflB; p2A4-ldh into Escherichia coli (E.coli MG1655) respectively to obtain recombinant bacteria 1, recombinant bacteria 3 and recombinant bacteria 6;
[0080] (4) Fermenting recombinant bacteria 1, recombinant bacteria 3 and recombinant bacteria 6 respectively to obtain recombinant bacteria 1 fermentation broth, recombinant bacteria 3 fermentation broth and recombinant bacteria 6 fermentation broth;
[0081] Fermentation process is with embodiment 1;
[0082] (5) The fermentation broth obtained in step (4) was centrifuged at 4°C and 6500rpm to collect the precipitate, and the precipitate was washed with 0.01M PBS (pH=7.5) to obtain the recombinant bacteria 1 fermentation broth precipitate, recombinant bact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com