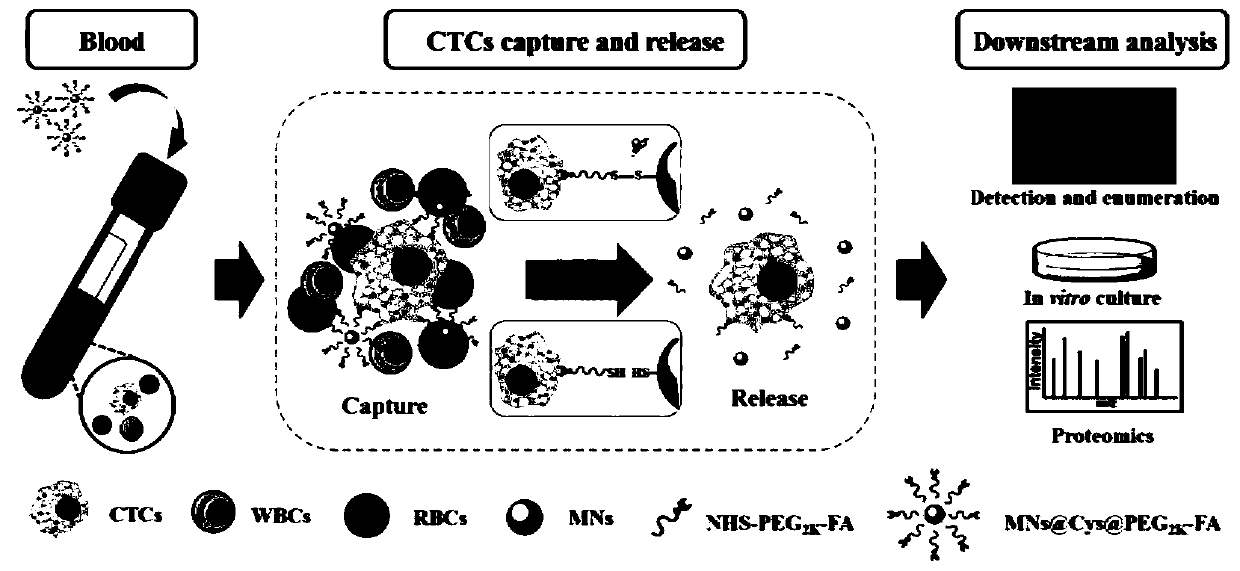
Kit for rapidly capturing, releasing and detecting circulating tumor cells without damage
A technology for detection kits and tumor cells, applied in the field of release and detection kits, rapid and non-damaging capture of circulating tumor cells, can solve the problems of high cost of antibodies, short shelf life, affecting the true characteristics of CTCs, etc., to improve prognosis and survival rate , fast magnetic response ability, controllable and easy coupling modification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The enrichment and separation of CTCs in the cell suspension comprises the following steps:
[0049] (1) When HEK293 cells were cultured to 70%-80% confluence, they were digested with trypsin and then pipet and mixed to prepare a single cell suspension (the concentration of the cell suspension was 10 6 / mL, monocytes in simulated blood), take 1mL and add it to a 2mL bovine serum albumin-coated centrifuge tube;
[0050] (2) Different numbers of pre-stained cervical cancer cells (Hela) were added to the HEK293 cell suspension to simulate CTCs;
[0051] (3) After mixing the cell suspension, add 100 μL of folic acid-modified CTCs magnetic nano-capture probe solution, place it on a rotary mixer and incubate at room temperature for 15 minutes;
[0052] (4) Then the centrifuge tube was placed in a 0.6T magnetic stand, magnetically separated for 2 minutes, and the supernatant was discarded;
[0053] (5) 100 μL of PBS resuspended magnetic nanoprobe-CTCs complex containing 1% B...
Embodiment 2
[0061] The capture of CTCs in blood includes the following steps:
[0062] (1) Take 1 mL of blood and add it to a 10 mL centrifuge tube, add pre-stained cervical cancer cells (Hela) into the blood to simulate CTCs, and pipette gently to mix;
[0063] (2) Add 3 mL of erythrocyte lysate to the centrifuge tube, let the centrifuge tube stand for 10 minutes, and shake it several times during the period;
[0064] (3) After mixing the cell suspension, add 100 μL of folic acid-modified CTCs magnetic nanocapture probe, place it on a rotary mixer and incubate at room temperature for 15 minutes;
[0065] (4) After the lysis, 2000rpm, 3min, discard the supernatant, resuspend the pellet in 1mL of PBS and transfer to a 2mL BSA-coated centrifuge tube;
[0066] (5) 100 μL of folic acid-modified CTCs magnetic nanocapture probe solution was added thereto, placed on a rotary mixer and incubated at room temperature for 15 minutes;
[0067] (6) Then the centrifuge tube was placed in a 0.6T magne...
Embodiment 3
[0076] The release efficiency verification of magnetically captured CTCs includes the following steps:
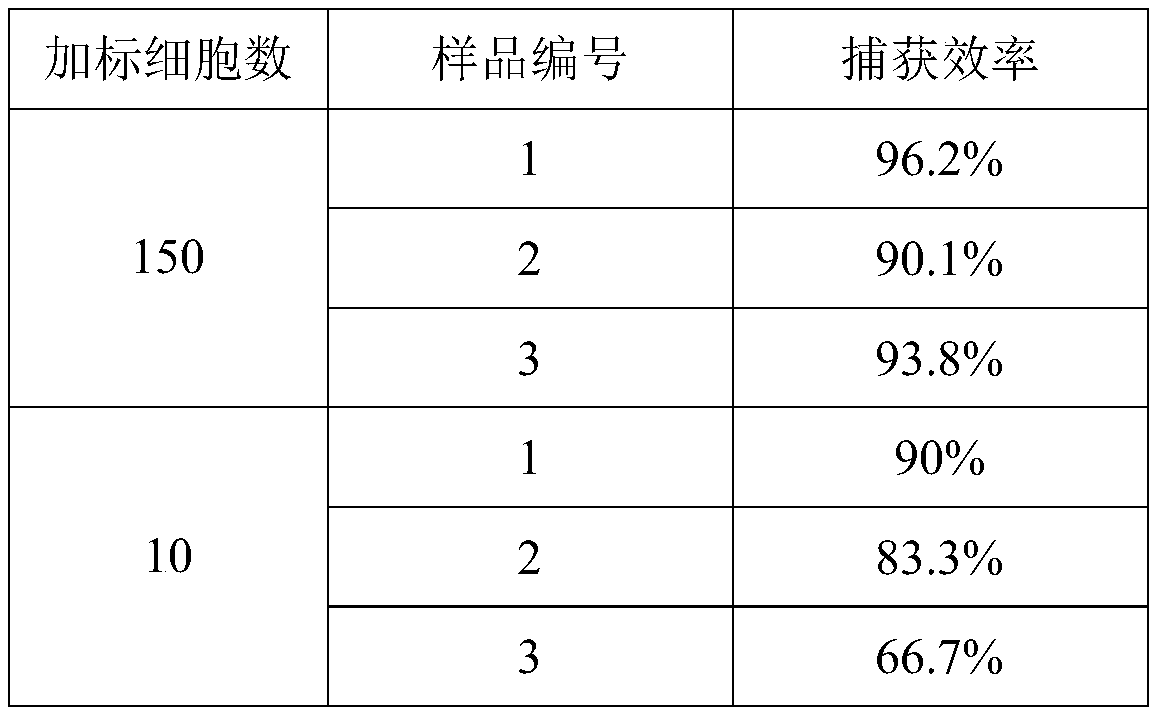
[0077] (1) In this example, about 200 pre-stained Hela cells were added to 1 mL of HEK293 cell suspension, and the centrifuge tube was inverted several times to mix well, and then 100 μL of CTCs magnetic nano-capture probe solution was added, and placed in a rotating mixture above Continue to incubate at room temperature for 15 minutes;
[0078] (2) Then the centrifuge tube was placed in a 0.6T magnetic stand, magnetically separated for 2 minutes, and the supernatant was discarded;
[0079] (3) 1mL of CTCs-containing release solution to resuspend the magnetic nanoprobe-CTCs complex should be placed in a constant temperature water bath at 37°C for continuous incubation for 30 minutes;
[0080] (4) Then the centrifuge tube was placed in a 0.6T magnetic stand, magnetically separated for 2 minutes, and the supernatant was discarded;
[0081] (5) 100 μL of PBS resuspended magn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com