Balloon uterine stent for preventing adhesion of uterine cavity
An anti-adhesion and balloon technology, applied in the direction of balloon-shaped catheters, catheters, coatings, etc., can solve the problems of no anti-bacterial and anti-infection, lack of visibility devices for uterine cavity anti-adhesion devices and instruments, and lack of endometrial repair and regeneration Active ingredients and other issues to achieve the effect of preventing cervical canal adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: The balloon uterine stent used for uterine cavity anti-adhesion of the present invention
[0069] The implementation mode of this embodiment is as follows:
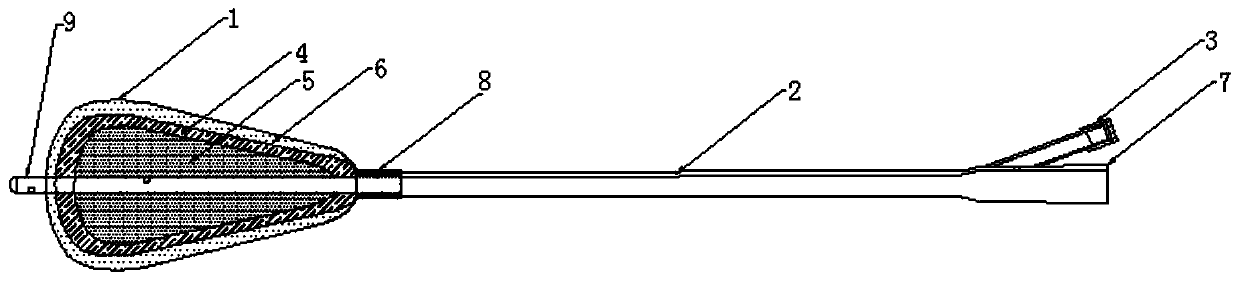
[0070] The balloon uterine stent for uterine cavity anti-adhesion is composed of a triangular balloon stent 1, a catheter 2, a one-way valve 3 and a drainage chamber 7;
[0071] Triangular balloon stent 1 is made of medical fluorosilicone rubber material, its balloon diameter is 30mm, its length is 30mm, and its capacity is 2ml;
[0072] One end of the catheter 2 is connected to the triangular balloon stent 1, the other end is connected to the drainage chamber 7, and the catheter 2 is connected to the drainage chamber 7;
[0073] The ratio of the diameter to the length of the conduit 2 is 1:18; the ratio of the length of the conduit 2 to the drainage chamber 7 is 1:12.
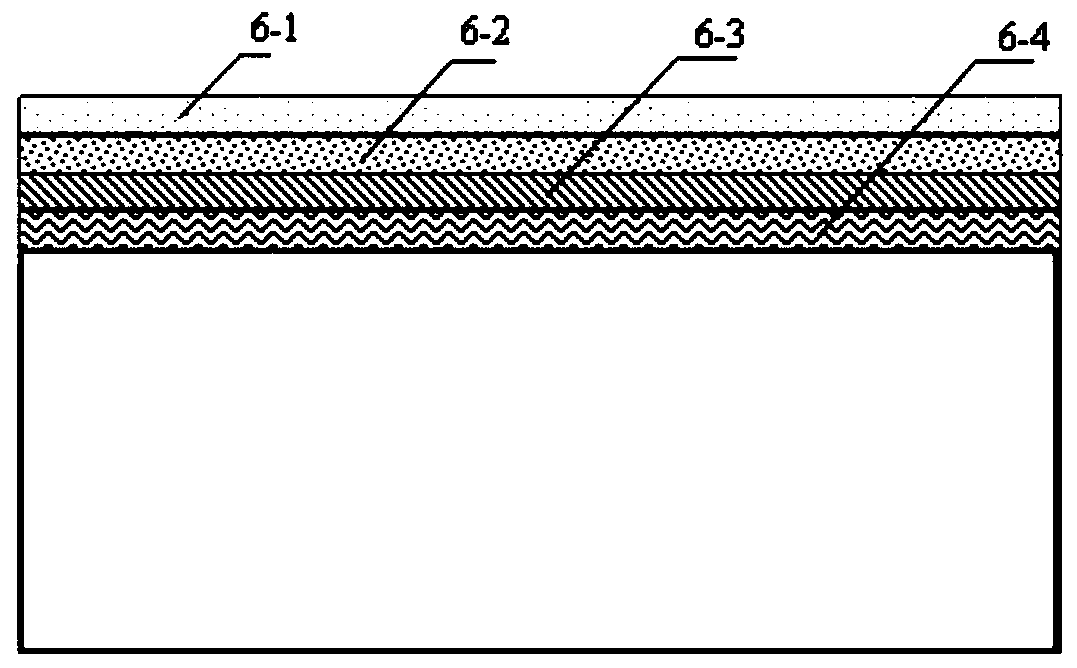
[0074] Barium sulfate developing lines 4 , pits 5 and functional coatings 6 are arranged on the surface of the triangular balloon ...
Embodiment 2
[0075] Embodiment 2: The balloon uterine stent used for uterine cavity anti-adhesion of the present invention
[0076] The implementation mode of this embodiment is as follows:
[0077] The balloon uterine stent for uterine cavity anti-adhesion is composed of a triangular balloon stent 1, a catheter 2, a one-way valve 3 and a drainage cavity 7;
[0078] Triangular balloon stent 1 is made of medical fluorosilicone rubber material, its balloon diameter is 35mm, its length is 35mm, and its capacity is 3ml;
[0079] One end of the catheter 2 is connected to the triangular balloon stent 1, and the other end is connected to the drainage cavity 7;
[0080] The ratio of the diameter to the length of the conduit 2 is 1:15; the ratio of the length of the conduit 2 to the drainage cavity 7 is 1:8.
[0081] Barium sulfate developing lines 4 , pits 5 and functional coatings 6 are arranged on the surface of the triangular balloon stent 1 . Wherein: the barium sulfate developing line 4 is a...
Embodiment 3
[0082] Embodiment 3: The balloon uterine stent used for uterine cavity anti-adhesion of the present invention
[0083] The implementation mode of this embodiment is as follows:
[0084] The balloon uterine stent for uterine cavity anti-adhesion is composed of a triangular balloon stent 1, a catheter 2, a one-way valve 3 and a drainage chamber 7;
[0085] Triangular balloon stent 1 is made of medical fluorosilicone rubber material, its balloon diameter is 40mm, its length is 25mm, and its capacity is 4ml;
[0086] One end of the catheter 2 is connected to the triangular balloon stent 1, and the other end is connected to the drainage chamber 7;
[0087] The ratio of the diameter to the length of the catheter 2 is 1:20; the ratio of the length of the catheter 2 to the drainage chamber 7 is 1:15.
[0088] Barium sulfate developing lines 4 , pits 5 and functional coatings 6 are arranged on the surface of the triangular balloon stent 1 . Wherein: the barium sulfate developing lin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Cross-sectional area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com