Autoantibody 7-AAb detection panel for hepatocellular carcinoma and application of autoantibody 7-AAb detection panel
A technology of hepatocellular carcinoma and autoantibodies, which is applied in the field of autoantibody 7-AAb detection panel of hepatocellular carcinoma, can solve the problems of non-specificity and achieve good diagnostic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 HuProt TM Human proteome microarray detects liver cancer and healthy control sera
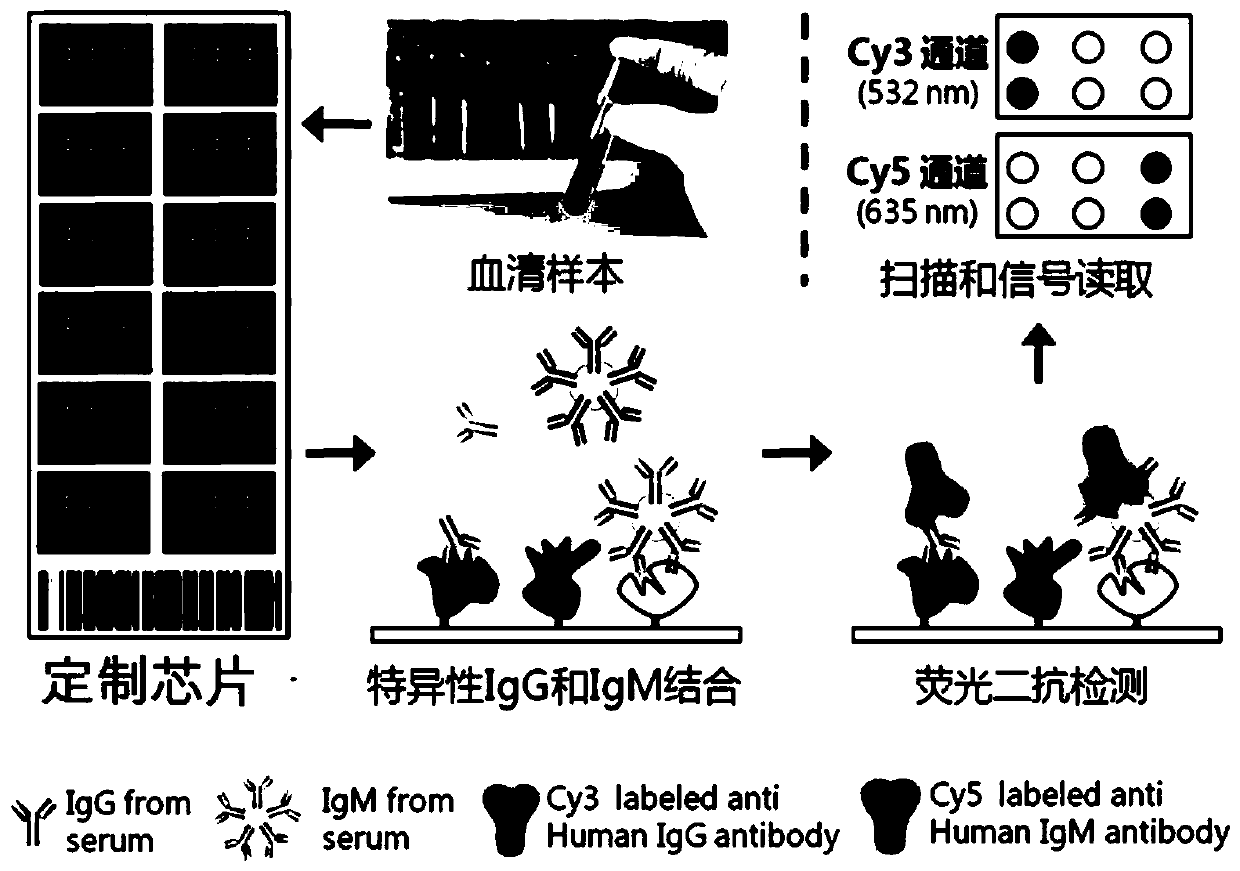
[0033] see process figure 1 Shown, specifically include the following steps
[0034] (1) Use 1 HuProt for each serum sample TM The proteome chip is used for detection, specific antibodies (including IgG, IgM) bind to the protein immobilized on the chip, wash to remove unbound antibodies and other proteins, and then use anti-human IgM fluorescently labeled secondary antibody (cy5 labeling, showing Red) and anti-human IgG fluorescent secondary antibody (cy3-labeled, green) detection, the signal is read by a fluorescent scanner, and the strength of the signal is positively correlated with the affinity and quantity of the antibody.
[0035] (2) A total of 50 cases of liver cancer and 50 cases of healthy control serum were detected. In order to eliminate errors caused by systematic differences between different samples and different chips, intra-chip and inter-chip normalization...
Embodiment 2
[0036] Example 2 Construction of HCC Focused Arrays
[0037](1) On the basis of normalization, statistical analysis was performed on the data to screen out specific high-response proteins of the liver cancer group. The analysis logic is as follows:
[0038] Parametric test t test, p-value<0.05 means that there is a significant difference between the two;
[0039] Calculate the multiple of difference between groups, when fold change ≥ 1.2, it is considered that there is a potential difference between the two;
[0040] Set cut off=mean+2SD (99%CI) for healthy group samples, and calculate the positive rate of HCC, with 10% as the minimum screening standard;
[0041] A total of 100 candidate biomarkers with certain discrimination ability or combined discrimination ability were screened out.
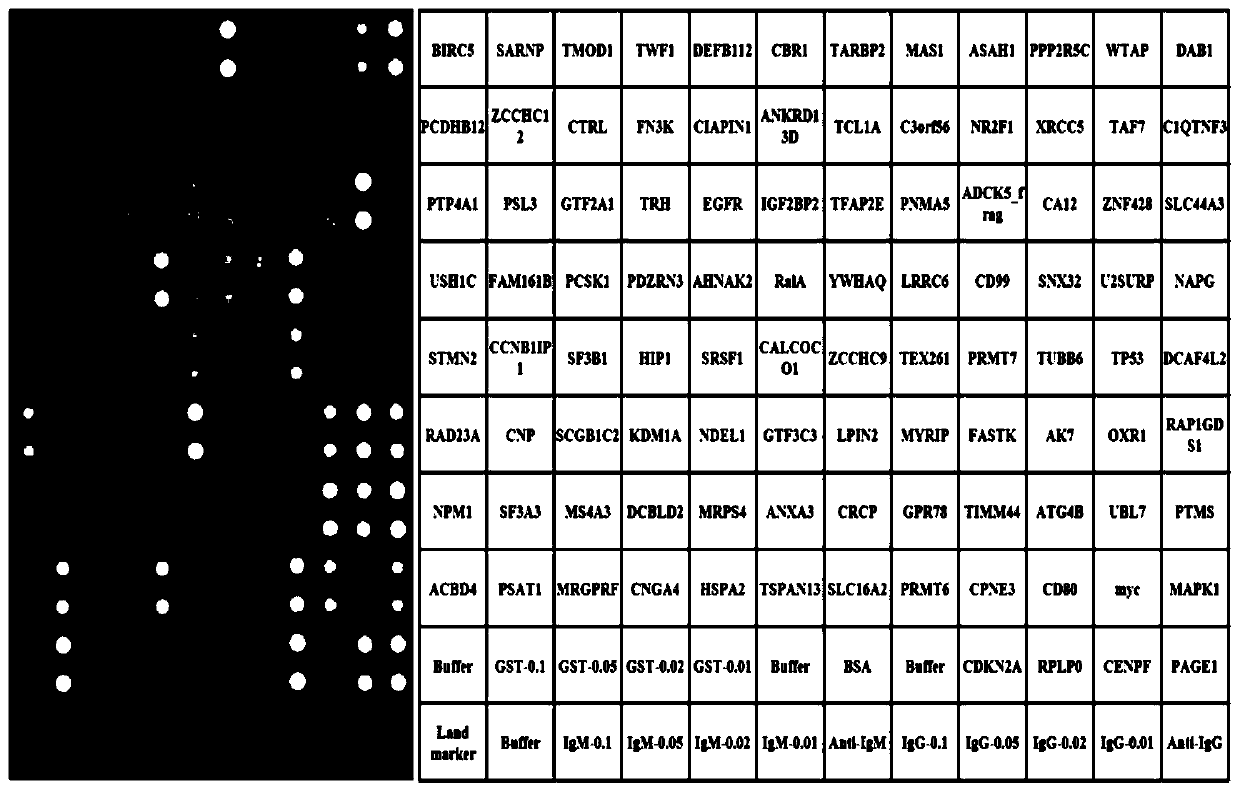
[0042] For the construction of HCC Focused Arrays and the list of 100 human recombinant proteins, see figure 2 .
[0043] (2) Use these 100 human recombinant proteins to make HCC Focused...
Embodiment 3
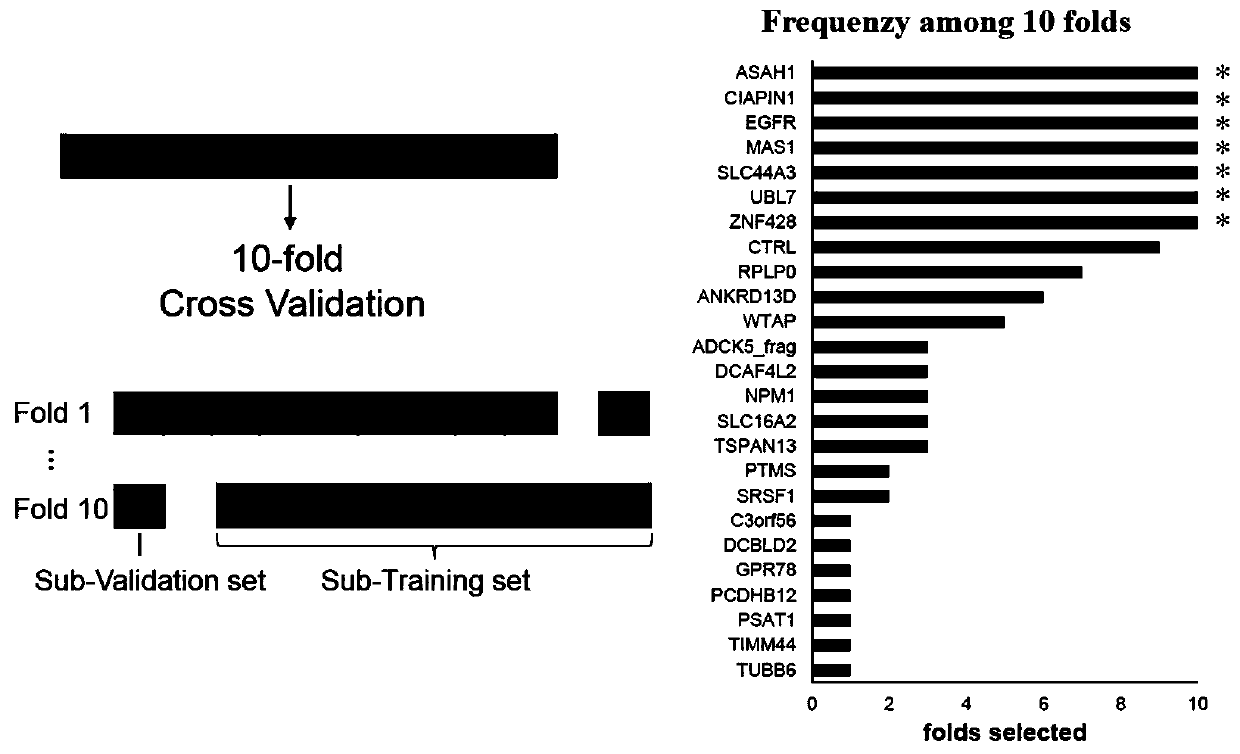
[0044] Example 3 Autoantibody 7-AAb Detection Panel Screening Process
[0045] Based on the small protein chip prepared in Example 2, we detected 561 cases of liver cancer patients and 592 cases of controls (including 343 cases of normal NC and 249 cases of LC of liver cirrhosis patients). Among them, 576 samples (282 liver cancer patients, 164 normal patients, and 130 liver cirrhosis patients) participated in model training (training set), and 577 samples (279 liver cancer patients, 179 normal patients, and 119 liver cirrhosis patients) were used as independent samples The model is validated by blind testing (validation set).
[0046] 1. Sample testing
[0047] 1) Rewarming: Take the chips out of the -80°C refrigerator, rewarm in a 4°C refrigerator for half an hour, and then rewarm at room temperature for 15 minutes.
[0048] 2) Sealing: After rewarming the chip, fix 14blocks fences, after fixing, add blocking solution to each block, place on a side swing shaker, and seal a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com