Blood stability nanoparticle-containing solution, preparation method and miRNA marker detection method thereof
A nanoparticle and marker technology, applied in the field of nanomaterials and medical nanoprobes, can solve the problems of inability to meet the early screening of clinical cancer, unstable test results, and high processing technology requirements, and achieve batch and automatic detection, The system has excellent blood stability and simple clinical operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
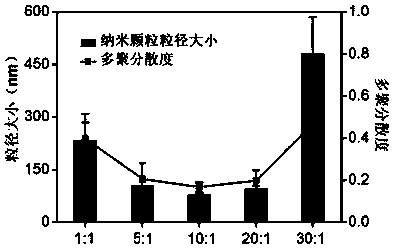
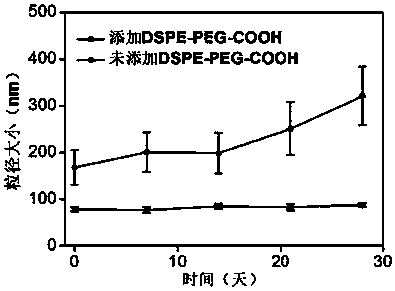
[0042] Dissolve 10 mg of PLGA-PEG-COOH (U.S. Creative PEGworks Company, product number DLG-5k20k21) and 1 mg of DSPE-PEG-COOH (U.S. Avanti Polar lipids company, product number 880135) in 2 mL of chloroform and mix well; Add dropwise to 4% ethanol aqueous solution, and use a cell ultrasonic breaker to continue ultrasonication at a frequency of 20kHz and a power of 90W for 5 minutes while adding, to promote the self-assembly of the two block copolymers and promote the volatilization of the chloroform solution to obtain polymer micelles solution.
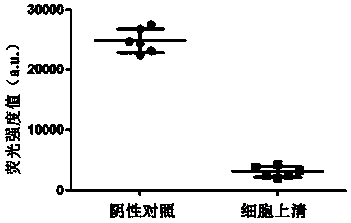
[0043] Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) (Merck sigma, Germany, product number E6383) and N-hydroxysuccinimide (NHS) (Germany) successively. Merck sigma company, Cat. No. 130672) (please add the amount of EDC and NHS), aminated complementary DNA at the 5' end of the corresponding cDNA (sequence 5'-GGGAGCTCATAGGCCGGCCCCTGGCTGCAGATG-3') of the custom-synthesized colorectal cancer marker miR-96-5p The ...
preparation Embodiment 2
[0045] Prepare the nanoparticle solution with reference to the preparation example 1 of the present invention, the difference is: the mass ratio of PLGA-PEG-COOH and DSPE-PEG-COOH is 5:1; ultrasonic treatment is performed with 60W power during the self-assembly process; coupled use Dialysis was performed using a dialysis bag with a molecular weight cut-off of 20 kD.
preparation Embodiment 3
[0047] Prepare the nanoparticle solution with reference to the preparation example 1 of the present invention, the difference is: the mass ratio of PLGA-PEG-COOH and DSPE-PEG-COOH is 20:1; ultrasonic treatment is performed with 130W power during the self-assembly process; coupled use Dialysis was performed using a dialysis bag with a molecular weight cut-off of 50 kD.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| surface potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com