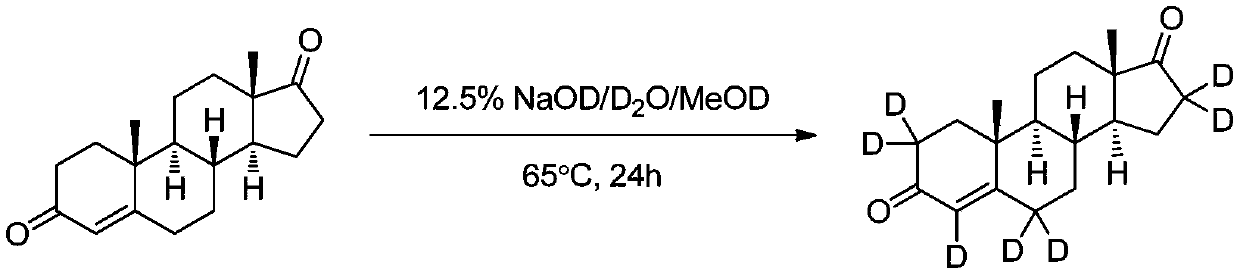
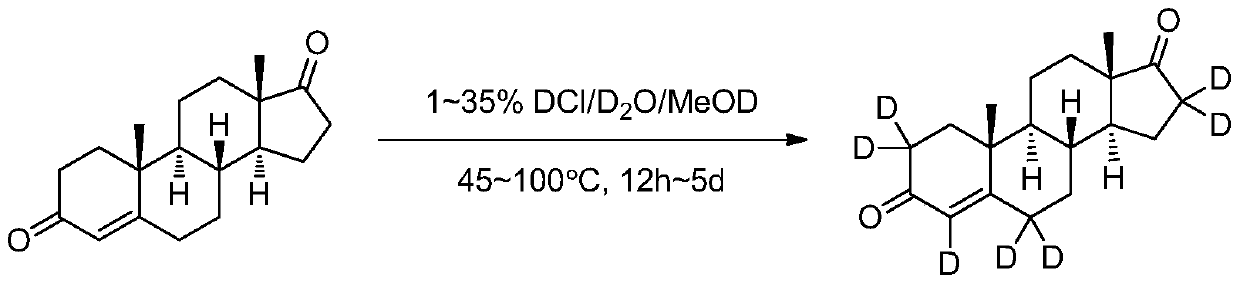
Method for synthesizing androstenedione (2,2,4,6,6,16,16-D<7>) through deuterium exchange
A technology for androstenedione and deuterium exchange, applied in organic chemistry methods, chemical instruments and methods, steroids, etc., can solve the problems of cumbersome treatment methods, cumbersome processes, and high costs, and achieve the effect of saving deuterated reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 100mg androstenedione to a 50ml single-necked flask connected with argon protection gas and a condenser tube, replace it with an oil pump three times, then add 1.5mL deuterated methanol-OD, after the dissolution is complete, slowly add 10% deuterated hydrochloric acid 5 mL of deuterium aqueous solution was heated to 55° C. under argon atmosphere, and the reaction was monitored by LC-MS, and the reaction was completed in about 10 to 12 hours. After the reaction, deuterated methanol (recyclable) was distilled off under reduced pressure, and the reaction solution was cooled, then left to refrigerate to crystallize, and a white solid was precipitated. Filter, dry, obtain about 85mg of product, about 85% of productive rate, product purity >98% (HPLC), LC-MS and 1 HNMR determined deuterium abundance >98%.
Embodiment 2
[0032] Add 200mg androstenedione to a 25ml single-necked flask connected with argon protective gas and a condenser tube, replace the oil pump three times, then add 1.5mL deuterated methanol-OD, after the dissolution is complete, slowly add 10% deuterated hydrochloric acid 3 mL of deuterium aqueous solution was heated to 55°C under argon atmosphere, and the reaction was monitored by LC-MS. After about 10 to 12 hours, the abundance stopped increasing. After the reaction, deuterated methanol (recyclable) was distilled off under reduced pressure, and the reaction solution was cooled, then left to refrigerate to crystallize, and a white solid was precipitated. Filter and dry, then add the same amount of deuterated methanol-OD and deuterated deuterium hydrochloride aqueous solution, continue to heat up and exchange, after 10 to 12 hours of reaction, spin dry deuterated methanol, refrigerate and crystallize, precipitate a white solid, filter and dry to obtain Product about 165mg, pro...
Embodiment 3
[0034] Add 980mg androstenedione to a 25ml single-necked flask connected with argon protective gas and a condenser tube, replace it three times with an oil pump, then add 3mL deuterated methanol-OD, after the dissolution is complete, slowly add 8% deuterated deuterated hydrochloride Aqueous solution 9mL, heated to 56°C under argon atmosphere, LC-MS to monitor the reaction, after the reaction was completed, deuterated methanol was evaporated under reduced pressure, and then the oil pump was spun to dry the water to obtain a solid, and then an equal amount of deuterated methanol was added- OD and deuterated deuterium hydrochloride aqueous solution, continue to heat up and exchange, perform three exchanges in total as above, after the last time, deuterated methanol is spin-dried, refrigerated and crystallized, a white solid is precipitated, filtered and dried to obtain about 910 mg of the product, and the yield is about 91%. Product purity>98.6% (HPLC), LC-MS and 1HNMR confirmed d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com