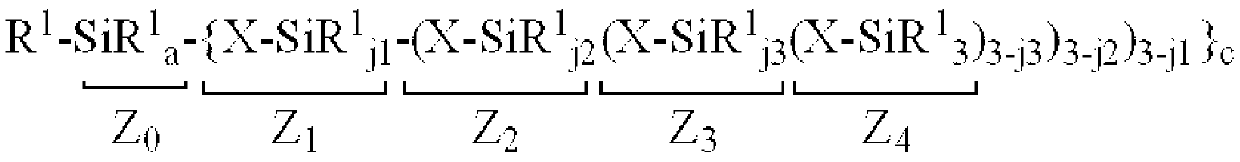Organopolysiloxane composition
A technology of polysiloxane and hydrogen polysiloxane, which is applied in the field of organopolysiloxane compositions, can solve the problems of high volatility, impracticality, and unsuitability for organosilicon materials, and achieve excellent solubility and practicability High, low odor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
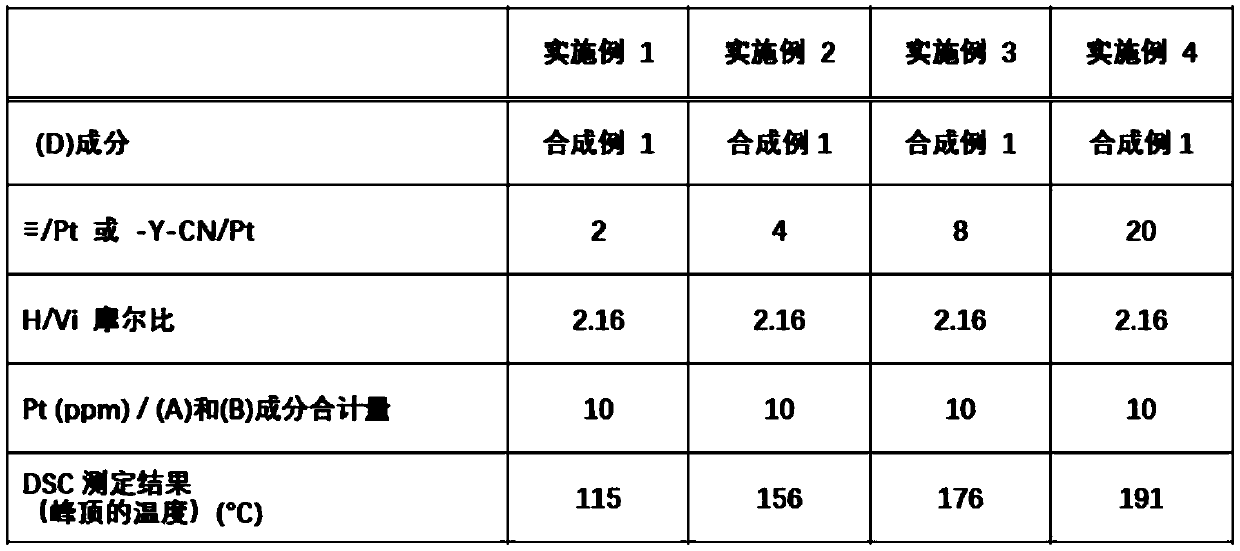
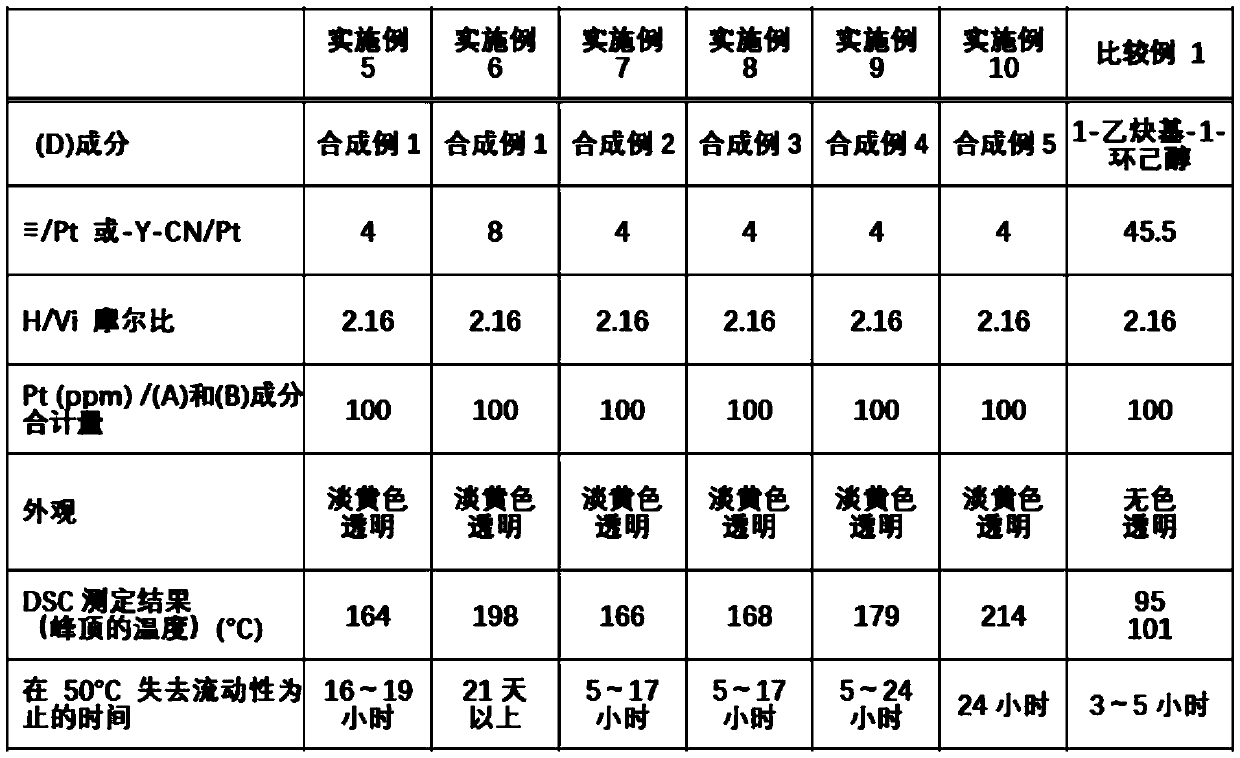
[0143] Examples and comparative examples are shown below to describe the present invention in detail, but the present invention is not limited by the following examples. In addition, Me represents a methyl group.
Synthetic example 1
[0145] 57.1 g (0.56 mol) of acetic anhydride was placed in a 300 mL flask, and the inner temperature was cooled to 5°C. 51.5 g (1.12 mol) of formic acid was added dropwise thereto. After further stirring for 30 minutes in a cooled state, the inner temperature was raised to 40° C., and after stirring for 2 hours, the mixture was cooled to room temperature to obtain a reaction liquid.
[0146] 106.0 g (0.30 mol) of 3-aminopropyltris(trimethylsiloxy)silane and 120.0 g of tetrahydrofuran were charged in a 500 mL flask, and the inner temperature was cooled to -15°C. The above-mentioned reaction solution was added dropwise thereto at such a rate that the internal temperature did not exceed -5°C. After completion of the dropping, stirring was further carried out at -15°C for 2 hours. Next, volatile matter was removed by an evaporator to obtain 118.2 g of a crude N-formylated product.
[0147] 118.2 g of the above-mentioned crude N-formylation product, 120.0 g of methylene chloride...
Synthetic example 2
[0149] 26.5 g (0.26 mol) of acetic anhydride was placed in a 300 mL flask, and the inner temperature was cooled to 5°C. 23.9 g (0.52 mol) of formic acid was added dropwise thereto. After further stirring for 30 minutes in a cooled state, the inner temperature was raised to 40° C., and after stirring for 2 hours, the mixture was cooled to room temperature to obtain a reaction liquid.
[0150] Fill in a 500mL flask n Bu(Me 2 )SiO(Me 2 SiO) 3 Si(Me 2 )CH 2 CH 2 CH 2 NH 2 65.4 g (0.14 mol) and 100.0 g of tetrahydrofuran, and the inner temperature was cooled to -15°C. The above-mentioned reaction solution was added dropwise thereto at such a rate that the internal temperature did not exceed -5°C. After completion of the dropping, stirring was further carried out at -15°C for 2 hours. Next, the volatile matter was removed by an evaporator to obtain 69.1 g of an N-formylated crude product.
[0151] 69.1 g of the aforementioned crude N-formylation product, 120.0 g of dichl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| rate of recovery | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| rate of recovery | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com