Electrochemical synthesis method of 2-substituted benzothiazole compound
The technology of a benzothiazole and a synthesis method, which is applied in the field of electrochemical organic synthesis, can solve the problems of increased operation difficulty, increased industrial cost, and high equipment requirements, and achieves the effects of low production cost, high product yield and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] Based on the above-mentioned reaction principle, the electrochemical synthesis method of the 2-substituted benzothiazole compounds of the present invention specifically includes the following steps:
[0038] (1) Add electrolyte, manganese salt catalyst, electrolytic solvent, benzyl ether compound and o-aminothiophenol in the electrolytic cell without diaphragm, insert anode and cathode, and carry out electrochemical reaction under stirring, electrification and constant current conditions;
[0039] Wherein, the molar ratio of the o-aminothiophenol to the benzyl ether compound is 1:1-1:1.5; the addition amount of the manganese salt catalyst is 10%-20% of the addition amount of the o-aminothiophenol; The molar concentration of the electrolyte in the electrolytic solvent is 0.1-0.2 mol / L; the electrochemical reaction conditions are: reaction time 2-4 hours, reaction temperature 45-60° C., and reaction current 15-30 mA.
[0040] And the electrolyte is any one or a combinatio...
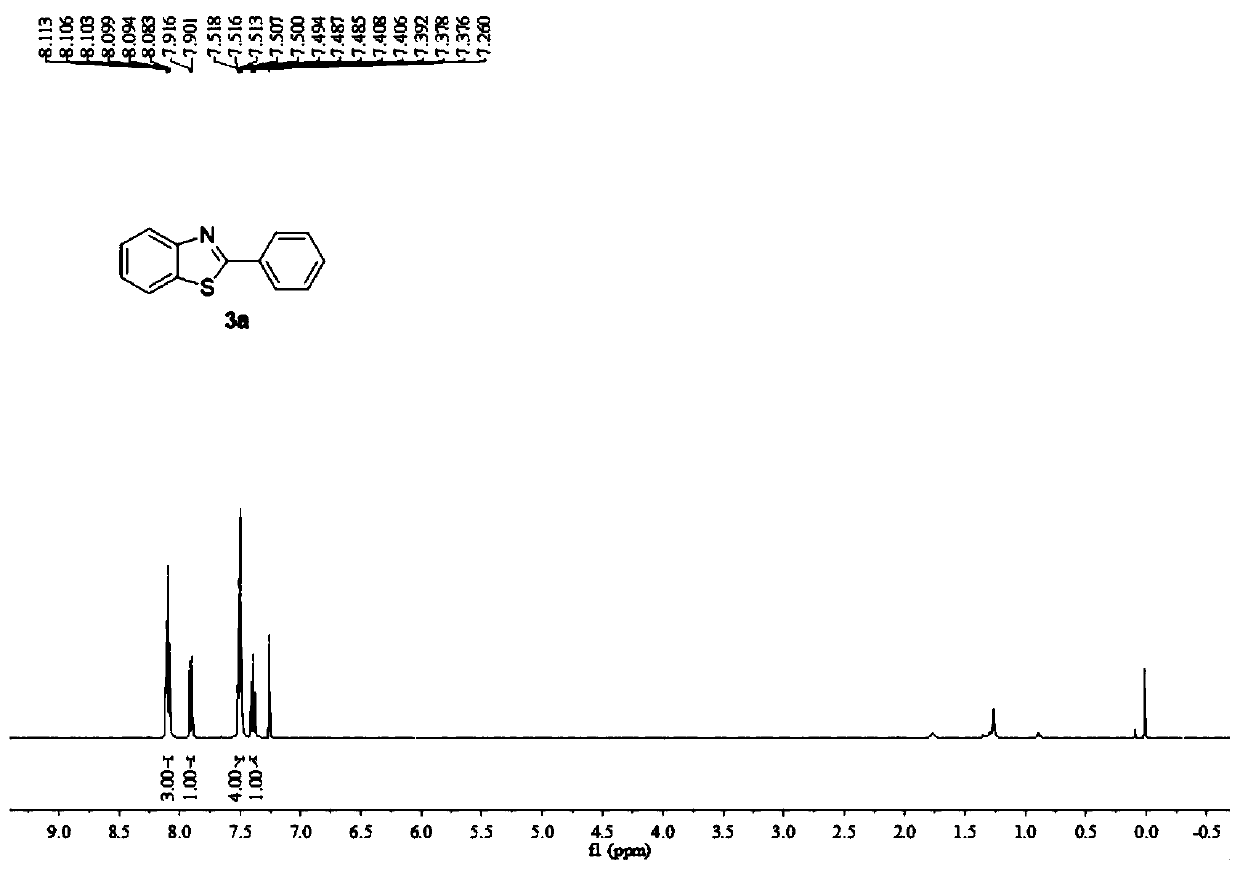
Embodiment 1
[0044] In Example 1, 2-phenylbenzothiazole (3a) was synthesized by electrochemical synthesis using benzyl methyl ether (1a) and o-aminothiophenol (2a) as raw materials. The reaction principle is as follows:
[0045]
[0046] Wherein, in the present embodiment 1, the anode is a platinum electrode (Pt), the cathode is a platinum electrode ((Pt), and the electrolyte is lithium perchlorate (LiClO 4 ), the manganese salt catalyst is manganese sulfate monohydrate (MnSO 4 ·H 2 O), the electrolytic solvent is acetonitrile (CH 3 CN) and acetic acid (HOAc).
[0047] Specifically, a platinum electrode was used as an anode and a platinum electrode was used as a cathode, and 1 mmol LiClO was sequentially added to a round bottom flask 4 , 0.02mmol MnSO 4 ·H 2 O, 0.75mmol benzyl methyl ether, 0.5mmol o-aminothiophenol, 10mL CH 3 CN and 400μL HOAc were put into a magnetic stirrer, the cap was closed, the power was turned on, the current was adjusted to 20mA, and the electrolysis was ...
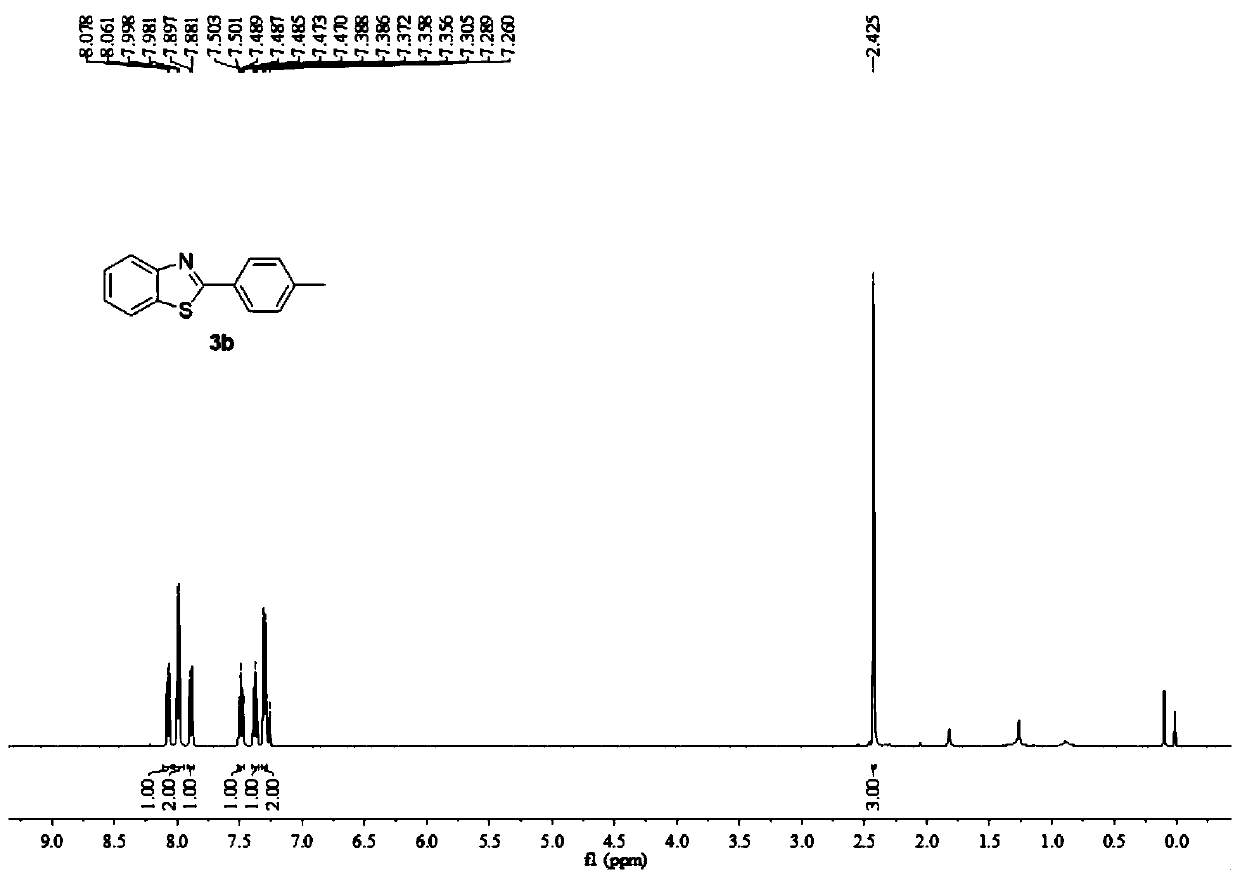
Embodiment 2
[0053] In Example 2, 2-(4-methylphenyl)benzothiazole (3b) was synthesized by electrochemical synthesis using benzyl tert-butyl ether (1b) and o-aminothiophenol (2a) as raw materials. The reaction principle is as follows:
[0054]
[0055] Wherein, in this embodiment 2, the anode is a platinum electrode (Pt), the cathode is graphite, and the electrolyte is ammonium perchlorate (NH 4 ClO 4 ), the manganese salt catalyst is manganese acetate tetrahydrate (Mn(OAc) 2 4H 2 O), the electrolytic solvent is acetonitrile (CH 3 CN) and trifluoroacetic acid (CF 3 COOH) mixture.
[0056] Specifically, a platinum electrode was used as the anode, graphite was used as the cathode, and 1 mmol NH 4 ClO 4 , 0.02mmol Mn(OAc) 2 4H 2 O, 0.75mmol benzyl tert-butyl ether, 0.5mmol o-aminothiophenol, 10mLCH 3 CN and 300 μL CF 3 COOH, put it into a magnetic stirrer, cover the bottle cap, turn on the power supply, adjust the current to 30mA, and electrolyze at 50°C for 2h. After the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com