Preparation method of trifluoromethyl tetralone compound
A technology of trifluoromethyltetralone and fluoromethyltetralone, which is applied in the field of preparation of trifluoromethyltetralone compounds, can solve the problems of large side effects, increased burden on the gastrointestinal tract, and slow curative effect, and achieves The effect of low cost, remarkable bactericidal and bacteriostatic effects, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
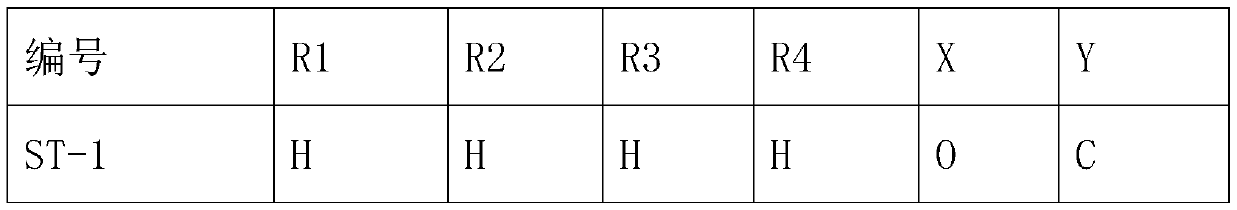
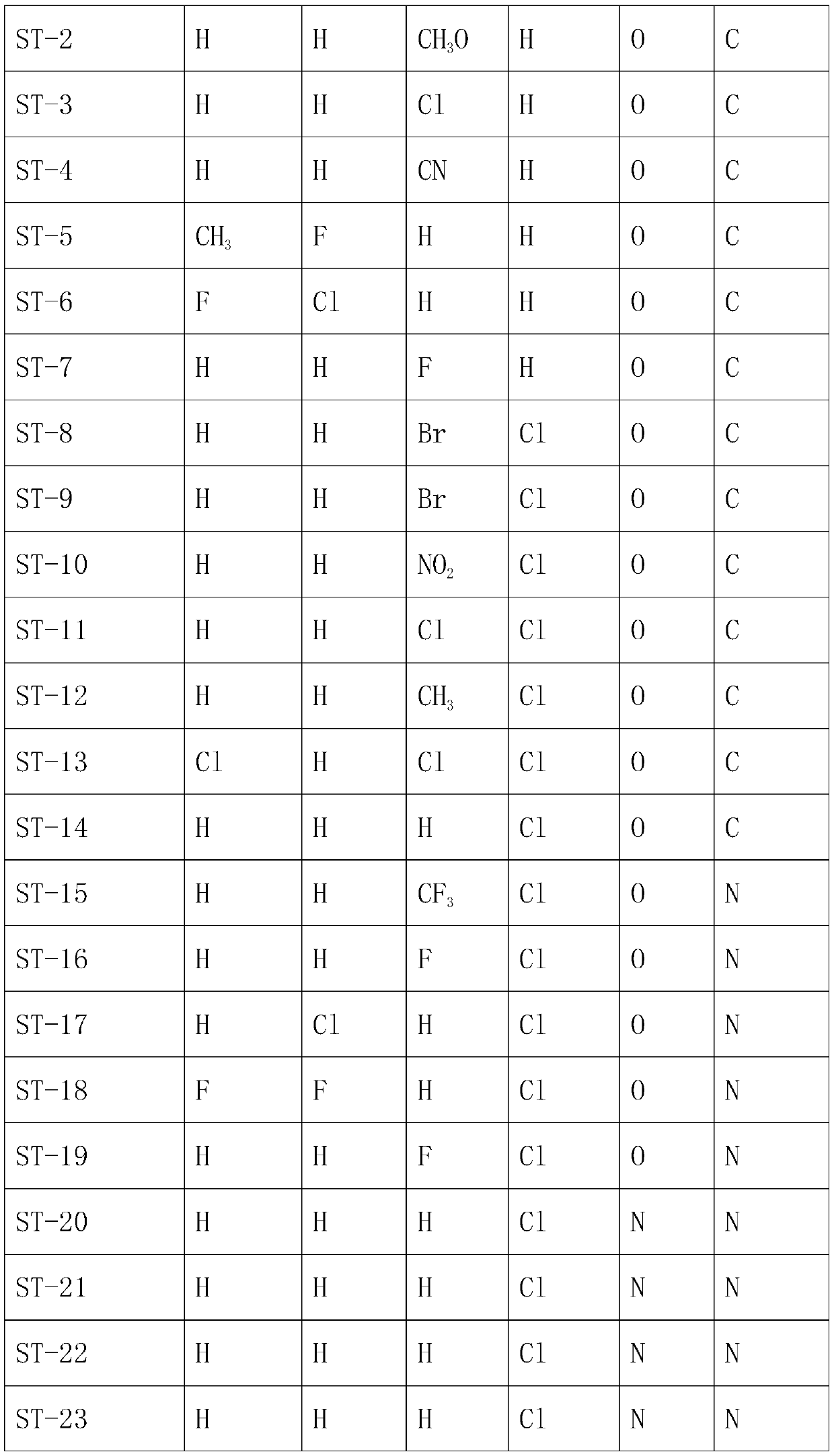
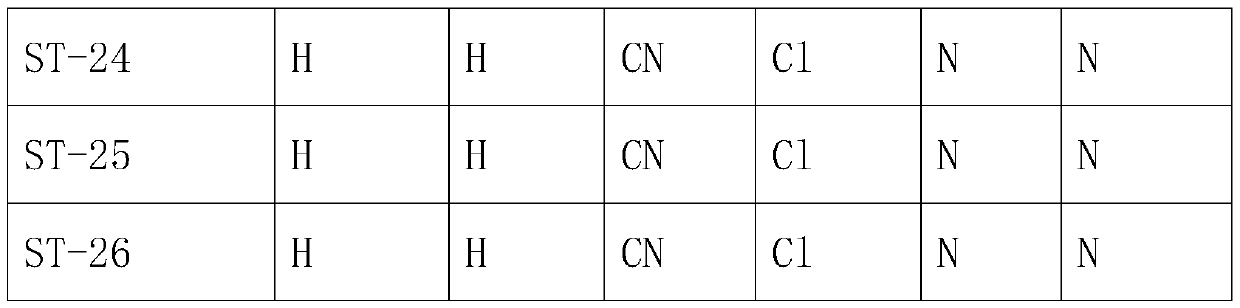
[0030] A preparation method of trifluoromethyl tetralinone compound, comprising the following steps:
[0031] S1. Take 17.61g (0.1mol) of 4-methoxytetralone and add it to a 250mL three-neck flask, then add 100Ml dichloromethane into it, add 28.21g (0.1mol) of trifluoromethanesulfonic anhydride under ice bath, and keep warm for 2h , quenched the reaction with saturated ammonium chloride, extracted with dichloromethane, and dried over anhydrous sodium sulfate to obtain product S215g, with a yield of 50.0%. Other products can be obtained in the same way.
[0032]S2. Take 31.01g (0.1mol) of S2 and put it into a 250mL three-necked flask, then add 100ml of acetonitrile into it, add 22.82g (0.1mol) of ammonium persulfate in the ice bath, react in the ice bath for 30min, remove the ice bath, and continue to stir for 2h , the reaction was monitored by TLC, the reaction was quenched with saturated ammonium chloride, extracted with dichloromethane, and dried over anhydrous sodium sulfat...
Embodiment 2
[0041] A preparation method of trifluoromethyl tetralinone compound, comprising the following steps:
[0042] S1. Take 16.1g (0.1mol) of 4-methyltetralone and add it into a 250mL three-neck flask, then add 100ml of dichloromethane, add 28.21g (0.1mol) of trifluoromethanesulfonic anhydride under ice bath, and keep it warm for 2h. The reaction was quenched with saturated ammonium chloride, extracted with dichloromethane, and dried over anhydrous sodium sulfate to obtain 13.2 g of product S21 with a yield of 45.2%.
[0043] S2. Take 29.2g (0.1mol) of S21 and add it to a 250mL three-necked flask, then add 100ml of acetonitrile into it, add 22.82g (0.1mol) of ammonium persulfate in the ice bath, react in the ice bath for 30min, remove the ice bath, and continue stirring for 2h , the reaction was monitored by TLC, quenched with saturated ammonium chloride, extracted with dichloromethane, and dried over anhydrous sodium sulfate to obtain product S35.72g, yield 25.3%.
[0044] S3. Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com