Anti-interleukin 17 (IL-17A) antibody and application thereof
An antibody and antigen technology, applied in the direction of antibodies, applications, antibody mimics/scaffolds, etc., can solve problems such as weak cell stability, complex expression regulation, tissue damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0153] Example 1. Recombinant protein human IL-17A-mFc
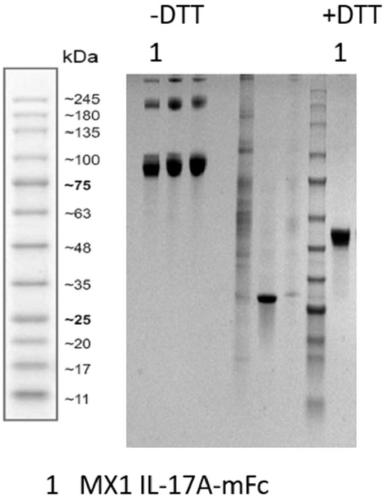
[0154] The plasmid HG12047-G encoding the full-length human IL-17A cDNA sequence (NCBI accession number: NP_002181.1) was purchased from Sino Biological, and the mature human IL-17A fragment (NCBI accession number NP_002181.1) was amplified by conventional PCR technology. The 24th-155th amino acid, the amino acid sequence is SEQ ID NO.:66, and the nucleotide sequence is SEQ ID NO.:67). After digestion with BSPQI, the amplified fragment was cloned into a self-constructed eukaryotic expression plasmid system (MXT1-Fc, containing the Fc domain of the murine IgG heavy chain) to generate the recombinant fusion protein expression plasmid IL-17A-mFc. Using conventional techniques, the correctly identified plasmid was transfected into expression cell 293F, expressed and purified to obtain human IL-17A-mFc recombinant protein. Such as figure 1 Shown is the SDS-PAGE electrophoresis of human IL-17A-mFc recombinant protein.
Embodiment 2
[0155] Example 2. Construction of 293F stably transfected cell lines expressing human IL-17RA
[0156] The plasmid HG10895-G containing the cDNA sequence encoding the full-length human IL-17RA was purchased from Sino Biological, and the DNA sequence encoding the full-length human IL-17RA (SEQ ID NO.: 69) was amplified by conventional PCR. Using conventional cloning techniques, the amplified fragments were cloned into a self-constructed eukaryotic expression plasmid system (HXP), which contains a puromycin screening system. The successfully constructed IL-17RA recombinant expression plasmid was transfected into 293F (ATCC) cells. After 24 hours of transfection, they were screened by puromycin (2 μg / ml) until the 293F IL-17RA stably transfected cell bank was formed. Use conventional methods to isolate single clones, such as by limiting dilution method, 0.8 cells per well, and spread 96-well plates. After 15 days, select IL-17RA-293F single clones and pass them to form 293F IL-1...
Embodiment 3
[0157] Example 3. Binding of recombinant protein IL-17A-mFc to 293F IL-17RA stably transfected cell line
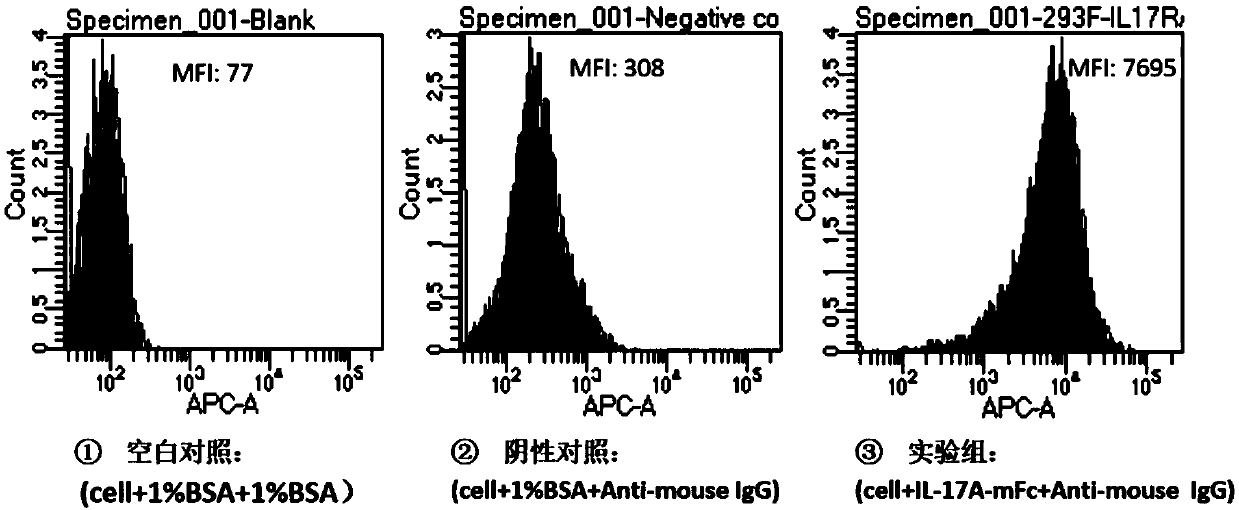
[0158] FACS experiment was used to detect the binding specificity of recombinant protein IL-17A-mFc to IL-17RA on 293F cells. In brief, cells (293F IL-17RA stably transfected cell line) were prepared into 1×10 6 / ml of cell suspension, add 20ul per well into a 96-well plate, the actual number of cells per well is 2×10 4 For each, the recombinant protein IL-17A-mFc (3ug / ml, 20ul / well; experimental group) or 1% BSA (20ul / well; negative control group) was mixed with the cell suspension, and after incubation at 37 degrees Celsius for 30 minutes, the FACS buffer After elution for 3 times, Anti-mouse IgG (1:200) was added and incubated at room temperature for 30 minutes. After elution with FACS buffer for 3 times, it was detected by flow cytometry, and the mean fluorescence intensity (MFI) of each group was compared. Such as figure 2 As shown, the recombinant protein IL-17...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com