A kind of fluorinated graphene modified niobium pentoxide material and its preparation and application
A technology of fluorinated graphene and niobium pentoxide, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of battery performance degradation, poor electronic conductivity, and shedding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] After mixing 45g of ethanol, 2g of fluorinated graphene (F / C ratio of 1.1, 5 layers), and 2g of ammonia water until uniform, slowly add 50g of niobium chloride, stir for 5 hours, and evaporate the liquid to obtain the intermediate phase. Then calcined at 600°C for 8 hours under argon atmosphere, and made into powder material after cooling.
[0020] The above materials were subjected to elemental analysis, wherein the mass fraction of carbon element accounted for 10%, the mass fraction of fluorine element accounted for 5%, and the rest was niobium pentoxide.
[0021] The above materials were tested by XPS and peaks were divided, of which 670.5e V belonged to Nb-F bond, 686.6e V belonged to C-F bond, and 675.3e V belonged to C-F-Nb bond, which proved that fluorinated graphene modified niobium pentoxide material Contains C-F-Nb bonds, Nb-F bonds, and C-F bonds. These bonds are conducive to the compounding of niobium pentoxide and graphene fluoride on the atomic scale to gr...
Embodiment 2
[0034] After mixing 45g of methanol, 3g of fluorinated graphene (F / C ratio of 1.2, 4 layers), and 2g of ammonia water until uniform, slowly add 50g of niobium fluoride, stir for 4.5 hours, and evaporate the liquid to obtain the intermediate phase. Then calcined at 800°C for 6 hours under nitrogen atmosphere, and made into powder material after cooling.
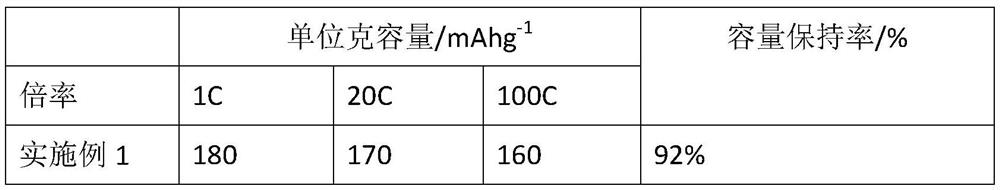
[0035] The above materials are used for electrode preparation and battery testing. The conditions for electrode preparation and battery testing are shown in the table.
Embodiment 3
[0037] Mix 80 g of isopropanol, 2.7 g of ammonia water, and 3 g of fluorinated graphene (F / C ratio of 1.1, 5 layers) until uniform, then slowly add 100 g of niobium bromide, stir for 3 hours, and evaporate the liquid to obtain intermediate Mutually. Then it was calcined at 500°C for 10 hours under an argon atmosphere, and it was cooled to make a powder material.
[0038] The above materials are used for electrode preparation and battery testing. The conditions for electrode preparation and battery testing are shown in the table.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com