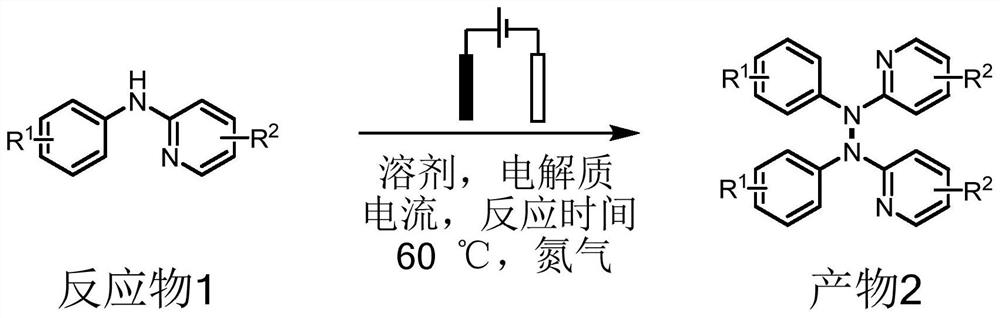
A method based on electrochemical synthesis of tetraarylhydrazines
A compound, the technology of arylhydrazine, applied in the field of electrochemical organic synthesis, can solve the problems of unfriendly environment, small scope of application of substrates, expensive catalysts, etc., and achieve simple and easy-to-obtain raw materials, wide application scope of substrates, and simple reaction system Efficient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add 0.25mmol of N-(4-chlorophenyl)-5-fluoropyridin-2-amine, 0.5mmol of tetrabutylammonium iodide as an electrolyte, 7.0mL of acetonitrile, and 0.5mL of methanol in a three-necked flask And a magnetic stirrer, nitrogen as a protective gas, a platinum sheet (1.0cm×1.0cm) as an anode and a cathode, turn on the power, adjust the current to 5.0mA, and react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain a tetraarylhydrazine compound with a yield of 99%. 2a.
[0061]
[0062] The product 2a that present embodiment obtains 1 H-NMR spectrum such as figure 2 as shown, 13 C-NMR spectrum such as image 3 As shown, the NMR data are as follows:
[0063] 1 H-NMR (400MHz, CDCl 3 ): δ8.11(d, J=2.6Hz, 2H), 7.39(d, J=8.7Hz, 4H), 7.30-7.24(m, 2H), 7.22(d, J=8...
Embodiment 2
[0067] Into a three-necked flask were sequentially added 0.25 mmol of 5-fluoro-N-(p-tolyl)pyridin-2-amine, 0.5 mmol of tetrabutylammonium iodide as an electrolyte, 7.0 mL of acetonitrile, 0.5 mL of methanol, and a magnetic Stirrer, nitrogen as protective gas, platinum sheet (1.0cm×1.0cm) as anode and cathode, power on, adjust current to 5.0mA, react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain 92% tetraarylhydrazine compound 2b, structural formula as follows:
[0068]
[0069] Product 2b 1 H-NMR spectrum such as Figure 4 as shown, 13 C-NMR spectrum such as Figure 5 As shown, the NMR data are as follows:
[0070] 1 H-NMR (400MHz, CDCl 3 ):δ8.09(d,J=2.8Hz,2H),7.35(d,J=8.4Hz,4H),7.25-7.19(m,2H),7.07(d,J=8.3Hz,4H),6.95 (dd,J=9.1,3.4Hz,2H),2.27(s,6...
Embodiment 3
[0074] 0.25mmol of N-(4-(tert-butyl)phenyl)-5-fluoropyridin-2-amine, 0.5mmol of tetrabutylammonium iodide as electrolyte, 7.0mL of acetonitrile, 0.5mL of methanol and a magnetic stirrer, nitrogen as a protective gas, platinum sheets (1.0cm×1.0cm) as anode and cathode, turn on the power, adjust the current to 5.0mA, and react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain a tetraarylhydrazine compound with a yield of 95%. 2c, the structural formula is as follows:
[0075]
[0076] Product 2c 1 H-NMR spectrum such as Figure 6 as shown, 13 C-NMR spectrum such as Figure 7 As shown, the NMR data are as follows:
[0077] 1 H-NMR (400MHz, CDCl 3 ): δ8.11(d, J=2.6Hz, 2H), 7.42(d, J=8.6Hz, 4H), 7.28(d, J=8.7Hz, 4H), 7.24-7.18(m, 2H), 6.96 (dd,J=9.1,3.3Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com