Fucose-rich exopolysaccharide as well as preparation method and application thereof
A technology of exopolysaccharides and fucose, applied in the field of exopolysaccharides, can solve problems such as the structure and application research of uncapsulated exopolysaccharides, and achieve the effect of strong application potential and strong antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, the preparation of microbial exopolysaccharide (EPS)
[0031] EPS is fermented and produced by Kosakonia sp.CCTCC M2018092 strain under fed-batch conditions. The specific steps are as follows: take Kosakonia sp.CCTCC M2018092 strain and culture it in a 250mL shake flask containing 30mL medium at 30°C and 200rpm for 20 hours , and then 30ml of bacterial culture solution was transferred to a 15L size fermenter at 30°C and 300rpm for pre-growth culture for 13 hours (air flow 1.5m 3 / h). Afterwards, get 3L pre-growth bacterial liquid and transfer in the 50L fermenter (containing 30L culture medium) to carry out fed-batch fermentation. According to the amount of residual sugar, a peristaltic pump was used to feed in 200 g / L glucose solution in batches at a rate of 0.9-3.8 rpm 13 hours after the fermentation started. The ventilation volume of the fermenter is 1.5m 3 / h, the dissolved oxygen concentration is controlled above 10% by automatically adjusting the s...
Embodiment 2
[0037] Embodiment 2, the structural identification of AH-EPS
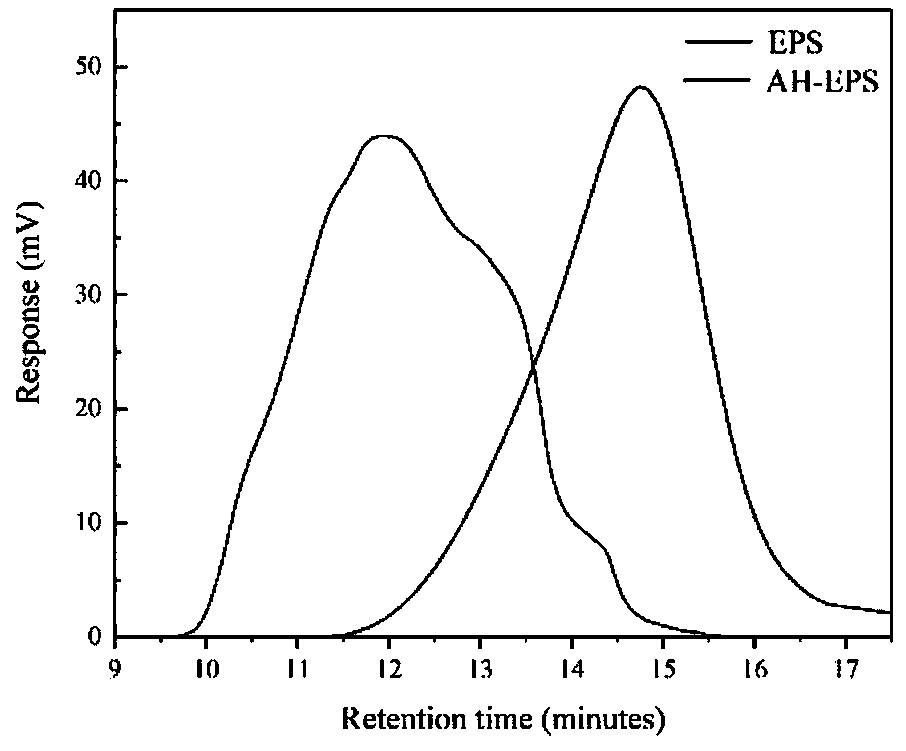
[0038] The weight average molecular weight (Mw) of EPS and AH-EPS was measured by gel permeation chromatography (GPC). A PL-GPC50 GPC Integrated System (Agilent) equipped with a PLAquagel-OH mixed-H 8 μm column and a differential detector was used. at 30 °C with 0.1 M NaNO 3 and 500ppm NaN 3 A sample with an appropriate concentration is separated as an eluent. The results showed that the EPS produced from Kosakonia sp.CCTCC M2018092 is a heterogeneous high molecular weight polysaccharide with a Mw of about 3.65×10 5 Da (Mw / Mn=1.7). AH-EPS is a homogeneous EPS with an average mass of 3.47×10 4 Da (Mw / Mn=1.2). Due to the advantages of large-scale production and homogeneity of AH-EPS, a comprehensive structural characterization of AH-EPS was carried out.
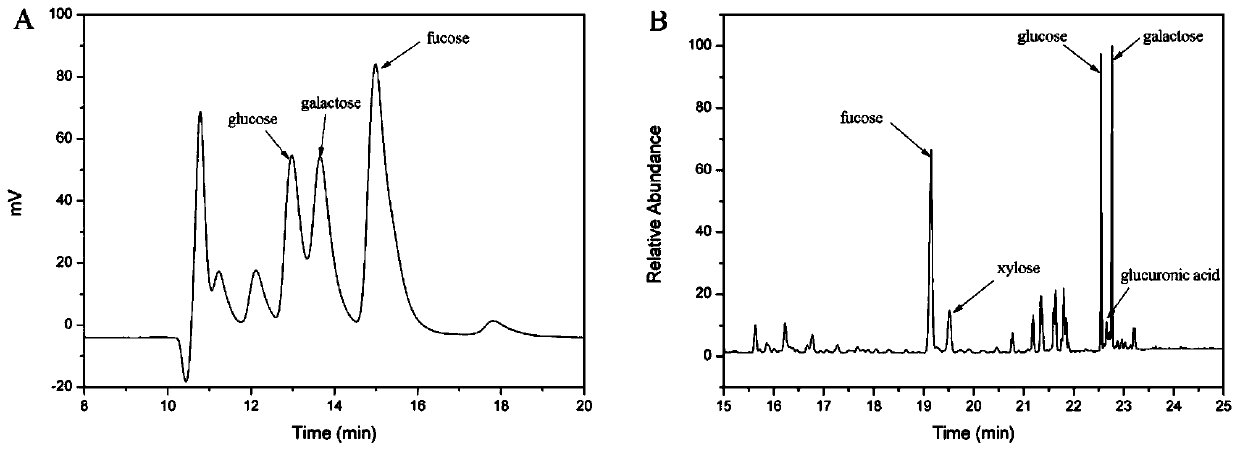
[0039] 1. High-performance liquid chromatography (HPLC) analysis of glycan composition
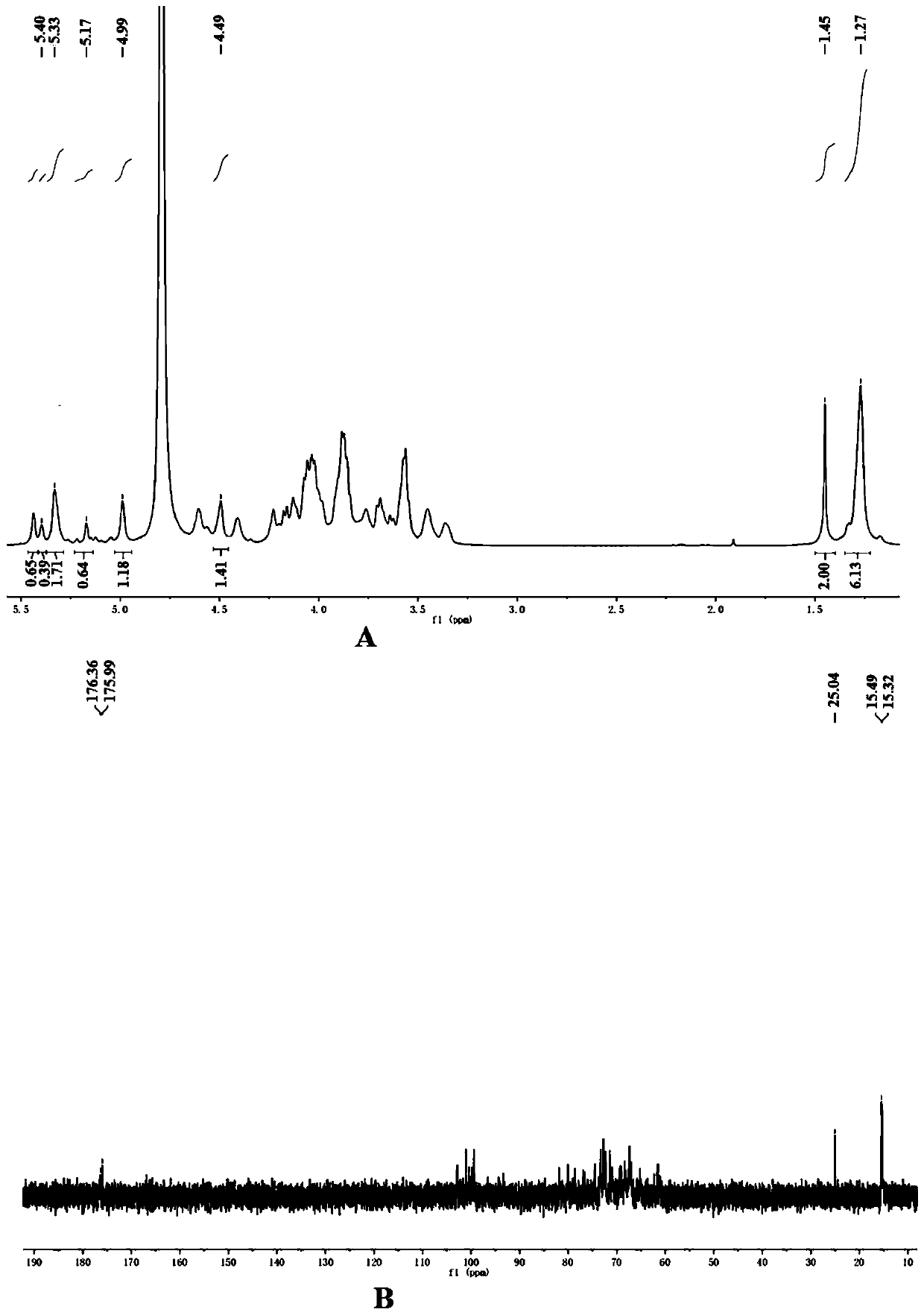
[0040] The monomer composition of AH-EPS was determined after compl...
Embodiment 3
[0073] Preparation and antibacterial test of embodiment 3, EPS / nanometer silver film
[0074] AH-EPS solutions with different concentrations (0.01mg / mL, 0.03mg / mL, 0.05mg / mL, 0.10mg / mL, 0.20mg / mL, 0.50mg / mL) and 2mM AgNO 3 The solution was mixed into 5 mL of water and shaken for 30 minutes. Afterwards, the solution was irradiated under a 354nm UV lamp for 12 minutes to prepare silver nanoparticles, and the nano-silver solution was added (0mL, 1.5mL, 3.0mL, 6.0mL) to 25mLEPS (1.5wt%) solution, and then the solution was poured Put it into a plastic flat plate (d=6.5cm), and form a film after drying at 50°C for 12h. Using the Kirby-Bauer method, the films were peeled off and cut into slice shapes (d=0.5 cm) for antibacterial testing, using Gram-positive Staphylococcus aureus (ATCC29213) as the test bacteria. The absorption spectra of silver nanoparticles solutions prepared by different concentrations of AH-EPS are as follows: Figure 9 Shown in A. The results showed that when...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com