Foamed nickel-based porous NiFe hydrotalcite nanosheet as well as preparation and application thereof
A technology of hydrotalcite and nanosheets, which is applied in the field of nanomaterial preparation and electrocatalysis, which can solve the problems of complex porous structure and lack of porous structure, and achieve excellent catalytic performance, simple operation and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
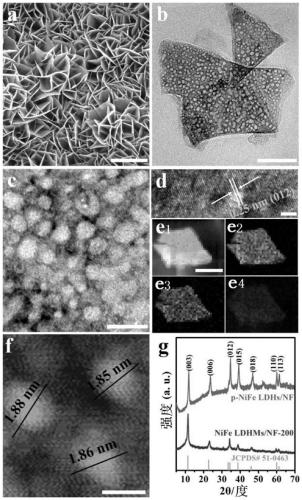
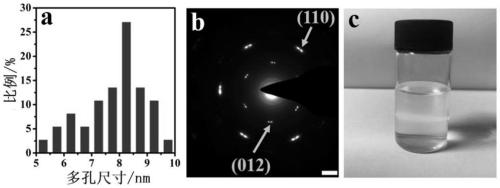
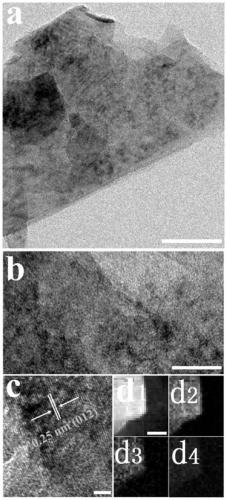
[0043] Embodiment 1: Preparation of foamed nickel-based porous NiFe LDH ultrathin nanosheet material (NiFe LDHMs / NF-200)
[0044] Weigh 0.214g (0.9mmol) of nickel chloride hexahydrate, 0.081g (0.3mmol) of ferric chloride hexahydrate, 0.498g (8.3mmol) of urea and 0.118g (3.2mmol) of ammonium fluoride and dissolve them in 20mL to In deionized water, stir to form a homogeneous solution. Add 200 μL of hydrogen peroxide solution dropwise to the above solution (the concentration of hydrogen peroxide solution is 0.0979 mol / L), and then transfer it to a 50 mL stainless steel reactor with a polytetrafluoroethylene liner, and place the treated nickel foam Immerse in the reaction solution prepared above, seal it and place it in an oven, react at 120°C for 16 hours, cool to room temperature naturally after the reaction, take out the reaction solution and centrifuge it at 10000rpm for 2min, and wash the obtained product with deionized water and anhydrous The porous NiFe LDH ultrathin nano...
Embodiment 2
[0050] Embodiment 2: Preparation of foamed nickel-based porous NiFe LDH ultrathin nanosheet material (NiFe LDHMs / NF-20)
[0051] Weigh 0.214g (0.9mmol) of nickel chloride hexahydrate, 0.081g (0.3mmol) of ferric chloride hexahydrate, 0.498g (8.3mmol) of urea and 0.118g (3.2mmol) of ammonium fluoride and dissolve them in 20mL to In deionized water, stir to form a homogeneous solution. Add 20 μL of hydrogen peroxide solution dropwise to the above solution (the concentration of hydrogen peroxide solution is 0.0979 mol / L), then transfer it to a 50 mL stainless steel reaction kettle with a polytetrafluoroethylene liner, and place the treated nickel foam Immerse in the reaction solution prepared above, seal it and place it in an oven, react at 120°C for 16 hours, cool to room temperature naturally after the reaction, take out the reaction solution and centrifuge it at 10000rpm for 2min, and wash the obtained product with deionized water and anhydrous The porous NiFe LDH ultrathin na...
Embodiment 3
[0052] Embodiment 3: the preparation (NiFe LDHMs / NF-60) of foam nickel-based porous NiFe LDH ultra-thin nanosheet material
[0053] Weigh 0.214g (0.9mmol) of nickel chloride hexahydrate, 0.081g (0.3mmol) of ferric chloride hexahydrate, 0.498g (8.3mmol) of urea and 0.118g (3.2mmol) of ammonium fluoride and dissolve them in 20mL to In deionized water, stir to form a homogeneous solution. Add 60 μL of hydrogen peroxide solution dropwise to the above solution (the concentration of hydrogen peroxide solution is 0.02937 mol / L), and then transfer it to a 50 mL stainless steel reactor with a polytetrafluoroethylene liner, and place the treated nickel foam Immerse in the reaction solution prepared above, seal it and place it in an oven, react at 120°C for 16 hours, cool to room temperature naturally after the reaction, take out the reaction solution and centrifuge it at 10000rpm for 2min, and wash the obtained product with deionized water and anhydrous The porous NiFe LDH ultrathin na...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com