Hydrogel for repairing peripheral nerve injury and preparation method of hydrogel
A peripheral nerve injury and hydrogel technology, applied in the field of biomedical engineering materials, can solve problems such as the effect of cross-linking agent PNI repair, and achieve the effect of prolonging the treatment period, achieving targeting, and definite curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] A kind of PLGA drug-loaded microspheres, the preparation method is as follows:
[0063] (1) Dissolve 0.3g of PVA in 300mL of deionized water, and dissolve it completely at 70°C to form a PVA solution;
[0064] (2) Dissolve 0.3g PLGA in 3.5mL dichloromethane solution to form PLGA solution;
[0065] (3) Dissolve NGF in PBS buffer solution to make 200ng / mL NGF solution, take 500 μL of NGF solution and drop into 3mL of PLGA solution to obtain PLGA / NGF mixed solution;
[0066] (4) Cool down the PVA solution and the PLGA / NGF mixed solution in an ice bath, take 500 μL of PBS buffer solution and add it to the PLGA / NGF mixed solution, and use an ultrasonic cell disruptor to ultrasonically disperse for 20 seconds at a power of 200 W until the liquid is milky white;
[0067] (5) Drop the above solution into 24mL PVA solution with a dropper, continue to ultrasonically disperse for 2min until the liquid is pale milky white, then place it on a magnetic stirrer and stir for 5h to com...
Embodiment 2
[0072] A CS-HEC-HA / GP hydrogel, the preparation method of which is:
[0073] (1) Stir and dissolve 40mg HA into 8mL distilled water to form HA solution;
[0074] (2) Add 20 mg of HEC into the HA solution, and continue to stir until the HEC is completely dissolved to form a mixed solution;
[0075] (3) Add 200mg CS into the mixed solution, stir evenly, add 1mL of 1mol / L hydrochloric acid solution, continue to stir until the CS is completely dissolved to form a CS / HEC / HA mixed solution, and store it at 4°C for later use;
[0076] (4) Slowly add 1 mL of 30% GP solution to 8 mL of CS / HEC / HA mixed solution dropwise, and continue to stir in the ice bath for 10 minutes to mix evenly;
[0077] (5) The above solution was poured into the mold, frozen in a refrigerator at -20°C, and dried in vacuum to obtain the CS-HEC-HA / GP hydrogel.
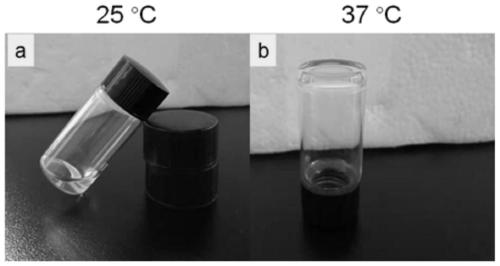
[0078] The schematic diagrams of gelation of the CS-HEC-HA / GP hydrogel prepared in this example at 25°C and 37°C are as follows image 3 shown. It ca...
Embodiment 3
[0081] A CS-HEC-HA(20) / GP(PLGA@NGF) hydrogel, comprising the following components in mass percent: 0.2% hyaluronic acid, 0.2% sodium hydroxyethylcellulose, 2% chitosan, 3% sodium β-glycerophosphate, 1% PLGA drug-loaded microspheres, and the balance is water. The preparation method of the PLGA drug-loaded microspheres is the same as in Example 1.
[0082] Its preparation method is:
[0083] (1) Stir and dissolve 20mg HA into 8mL distilled water to form HA solution;
[0084] (2) Add 20 mg of HEC into the HA solution, and continue to stir until the HEC is completely dissolved to form a mixed solution;
[0085] (3) Add 200mg CS into the mixed solution, stir evenly, add 1mL of 1mol / L hydrochloric acid solution, continue to stir until the CS is completely dissolved to form a CS / HEC / HA mixed solution, and store it at 4°C for later use;
[0086](4) Slowly add 1mL of 30% GP solution into 8mL CS / HEC / HA mixed solution dropwise. After the addition is completed, continue to stir in an i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
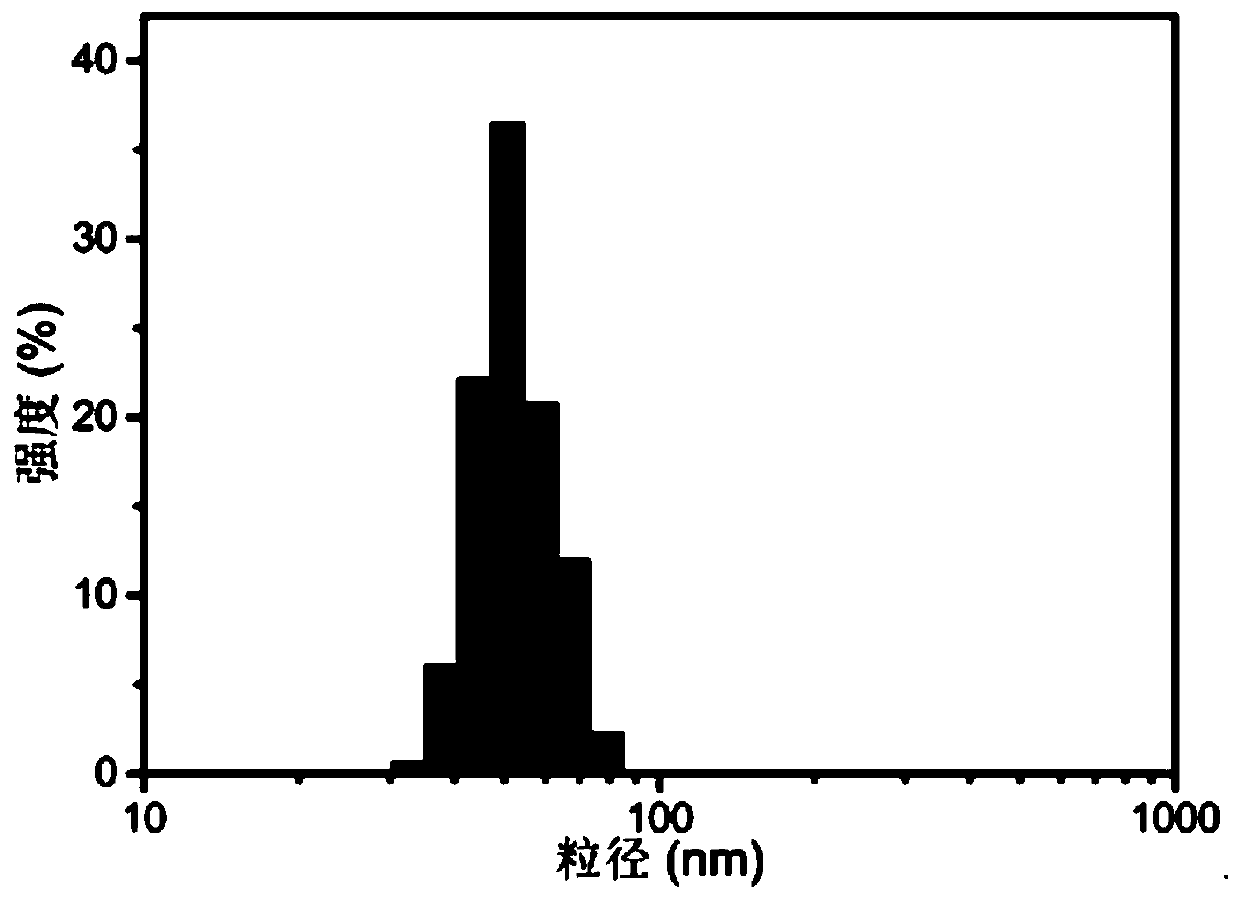
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com