Novel preparation for treating triple-negative breast cancer
A triple-negative breast cancer and preparation technology, applied in the direction of medical preparations containing active ingredients, organic active ingredients, drug combinations, etc., can solve the problems of lack of treatment options and insensitivity of triple-negative breast cancer, and achieve good clinical application prospects , Inhibition of cell proliferation, migration and invasion inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation method of miR-200c-3p agomir of the present invention
[0030] Synthesize double-stranded nucleic acid according to the sequence of miR-200c-3p, the 3' end base of the sense strand is modified with cholesterol, the 5' end is modified with two bases, and the 3' end is modified with 4 bases, antisense The entire base of the chain was methylated to obtain miR-200c-3pagomir.
[0031] Among them, the sequence of miR-200c-3p is:
[0032] UAAUACUGCCGGGUAAUGAUGGA (SEQ ID NO. 1).
[0033] The beneficial effect of the present invention will be further illustrated by means of experimental examples below. The materials involved in the experiment example are as follows:
[0034] 1 Cell lines and experimental mice
[0035] Experimental mice: 5-week-old non-obese diabetic / severe combined immune deficiency (NOD / SCID) female mice weighing 18 grams were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.
[0036] Cell line: triple-nega...
experiment example 1
[0050] Experimental Example 1 Cell Experiment
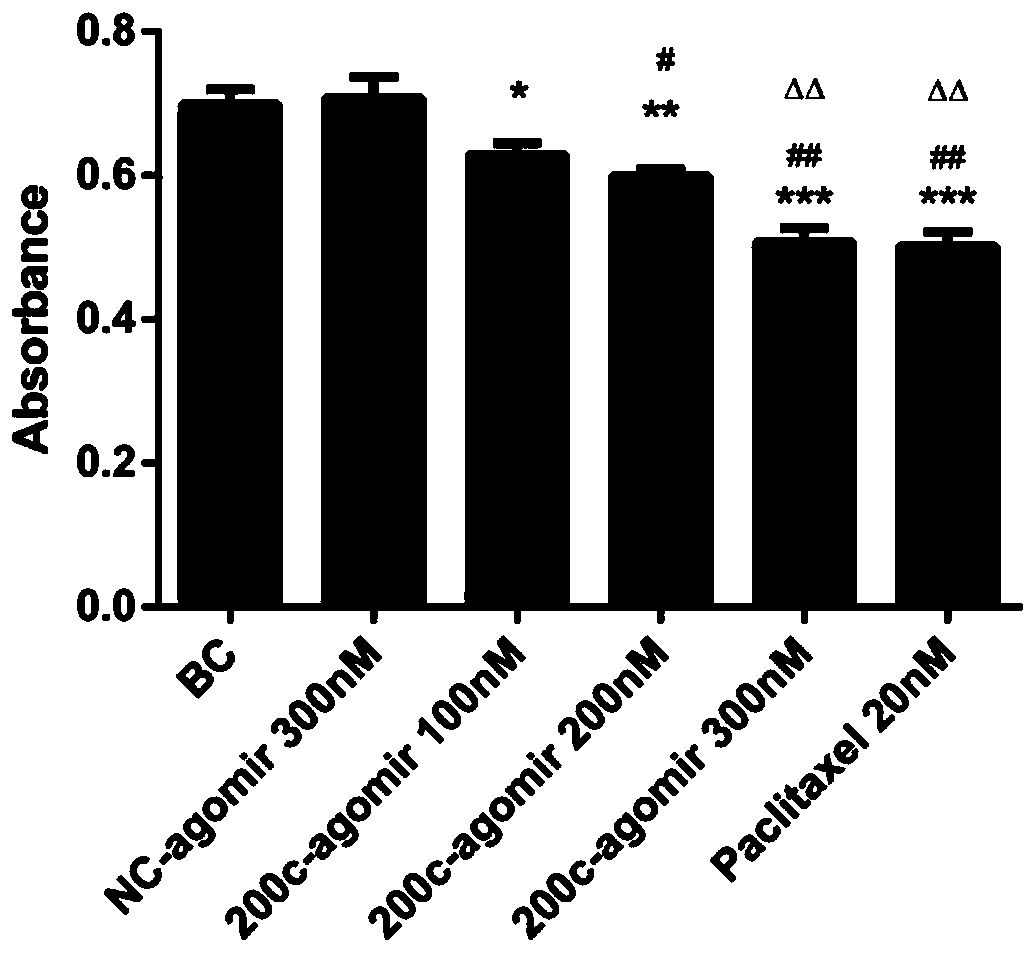
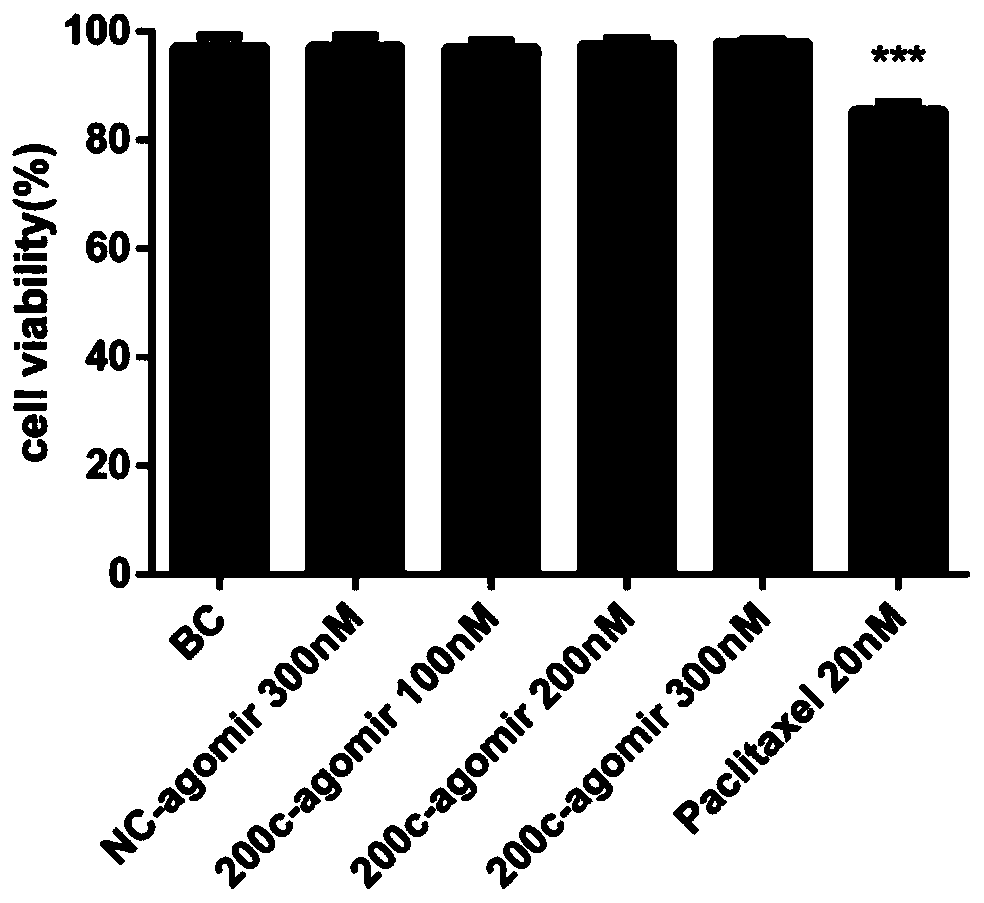
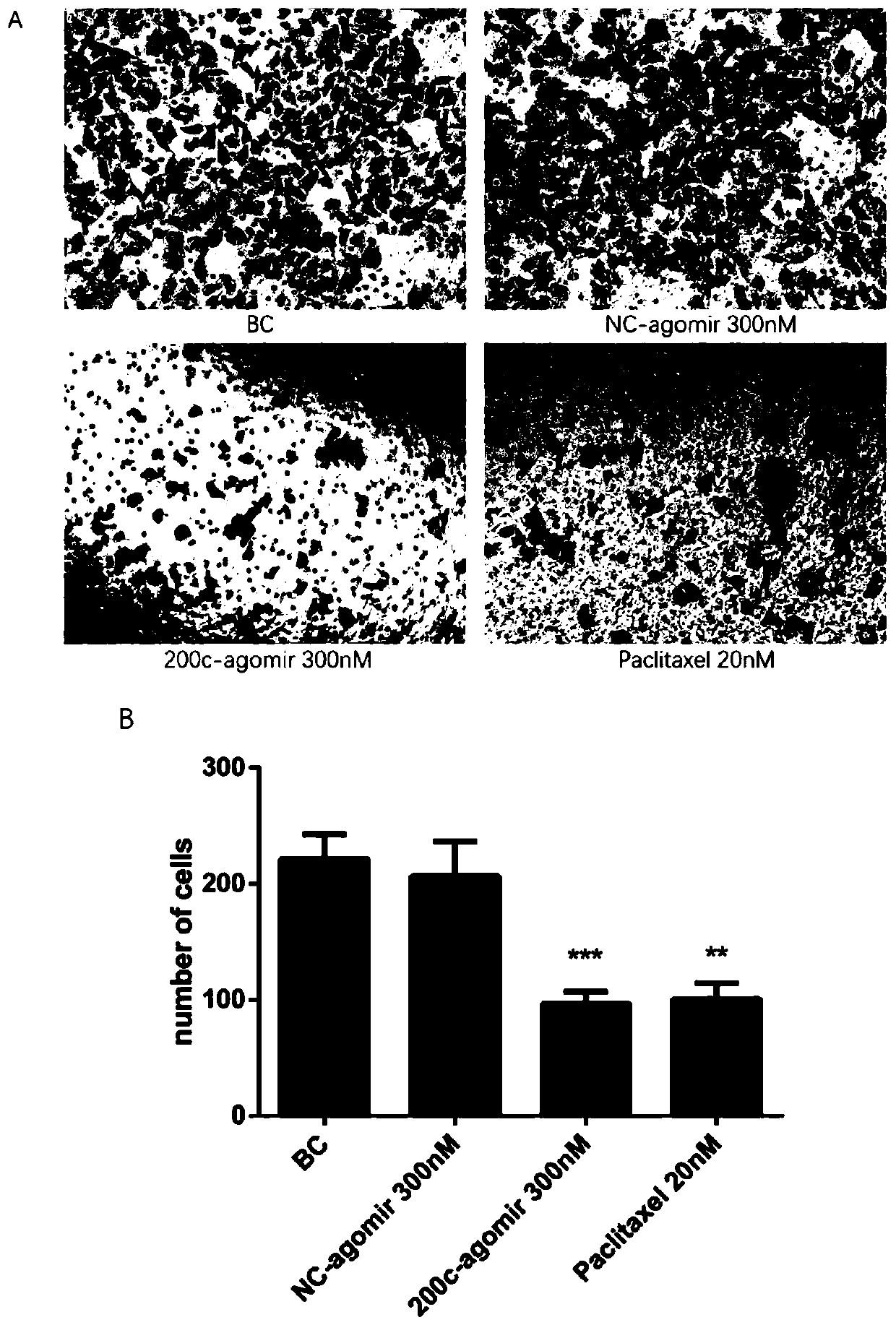
[0051] 1 miR-200c-3p agomir proliferation inhibition experiment
[0052] MB-231 cells were treated with miR-200c-3p agomir (100-300nM) and paclitaxel (20nM). After 48 hours of administration, the proliferation experiment of thiazolium blue (MTT) was performed to detect the light absorption value (OD value), OD value The lower the value, the more obvious the inhibition of cell proliferation.
[0053] Proliferation experiment steps are as follows:
[0054] 1) MB-231 cells in the logarithmic growth phase were digested with trypsin and then prepared into a single cell suspension with a culture medium containing 10% fetal bovine serum. The number of 5,000 cells per well was inoculated into 96-well culture plates, and 5 replicate wells were set up in each group.
[0055] 2) Place the 96-well culture plate at 37°C with 5% CO 2 Incubate for 24 hours in a cell culture incubator.
[0056] 3) The specific drug concentration given by ea...
experiment example 2
[0090] Experimental Example 2 Transplanted Tumor Inhibition Experiment
[0091] In order to further study the effect of miR-200c-3p on triple-negative breast cancer in vivo, the inventors established xenograft tumor models in non-obese diabetic / severe combined immunodeficiency (NOD / SCID) female mice, and injected miR-200c-3p related formulation to observe its effect on tumor growth. Among them, the inventors used two miR-200c-3p preparations, one is miR-200c-3p agomir, and the other is adeno-associated virus (adeno-associated virus2 / 9, AAV2 / 9) expressing miR-200c-3p. agomir is a microRNA that has been specially chemically modified (cholesterol modification at the 3' end, two thiol modifications at the 5' end, four thiol modifications at the 3' end, and full base methylation modification on the antisense strand), which can be directly used in Injected in animals to simulate endogenous mature microRNA; adeno-associated virus is an efficient delivery vehicle for gene therapy, wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com