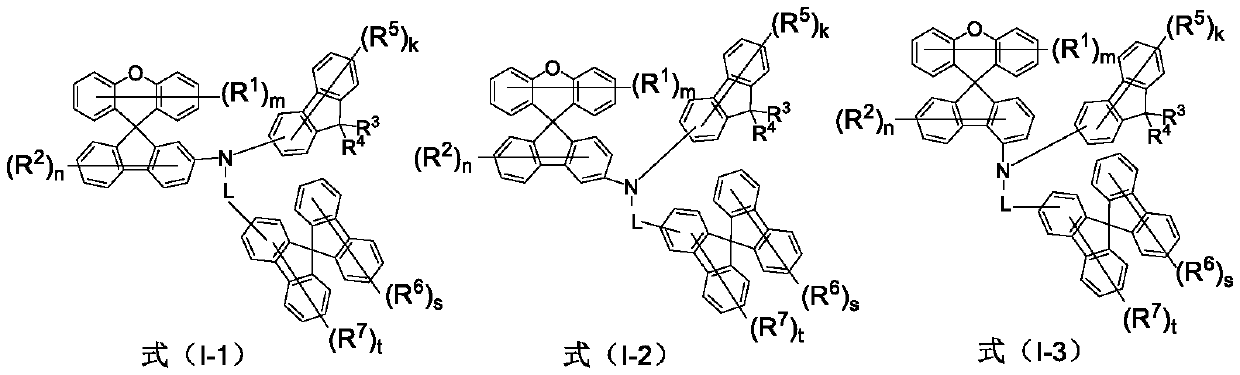
Amine derivative containing spirofluorene group and application of amine derivative in organic electroluminescent device
A technology of amine derivatives and spirofluorenyl, which is applied in the field of organic optoelectronic materials and devices, can solve the problems of low heat resistance, lower driving voltage, unsatisfactory current efficiency, etc., and achieve good hole transport ability, Reduced driving voltage, improved luminous efficiency and heat resistance effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
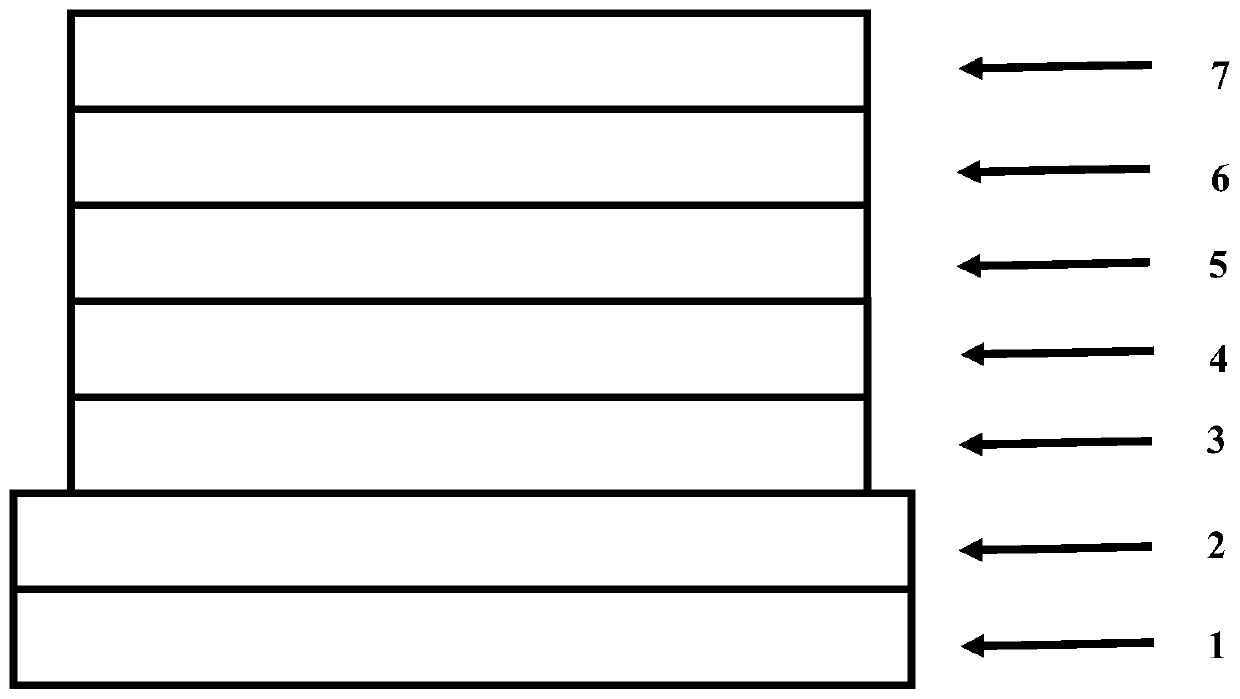
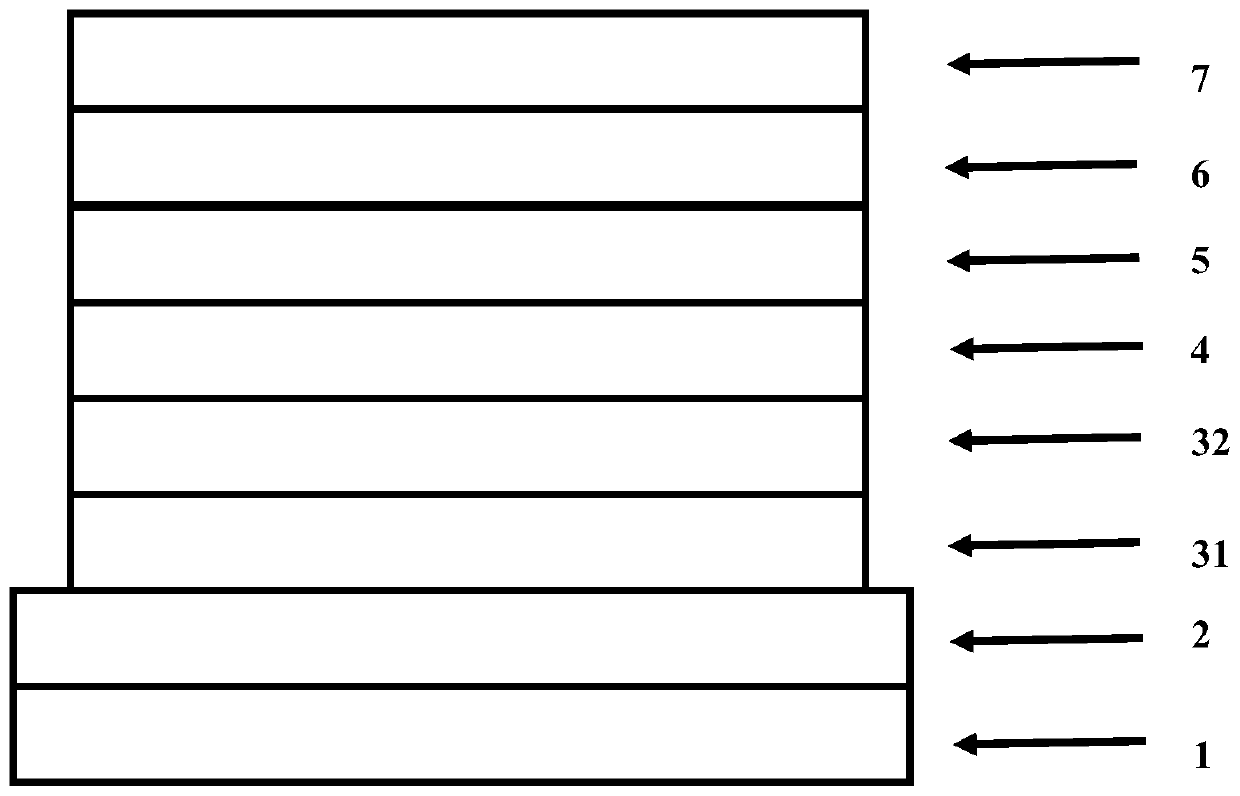
Image
Examples
Embodiment 1
[0063] The concrete preparation method of intermediate (I) is as follows:
[0064] Under a nitrogen atmosphere, add raw material (a) (15mmol), raw material (b) (15mmol), sodium tert-butoxide (30mmol) into 150mL of anhydrous toluene, add palladium acetate (0.3mmol), tri-tert-butyl Phosphine (0.3 mmol) was reacted at 80°C for 8 hours. After cooling, filter through a diatomaceous earth / silica gel funnel, remove the organic solvent from the filtrate by distillation under reduced pressure, recrystallize the obtained residue in toluene, collect the obtained solid by filtration, and dry to obtain intermediate (I). The structures of the obtained intermediates are shown in Table 1.
[0065] Taking intermediate 1 as an example to illustrate the specific details of the synthesis example: under nitrogen atmosphere, raw material a-1 (3.14g, 15mmol), raw material b-1 (5.91g, 15mmol), sodium tert-butoxide (2.88g, 30mmol ) into 150 mL of anhydrous toluene, palladium acetate (0.07 g, 0.3 mmo...
Embodiment 2
[0072] The concrete preparation method of compound is as follows:
[0073] Under nitrogen atmosphere, put starting material c-A (19.55mmol), intermediate (I) (20.14mmol) and sodium tert-butoxide (27.0mmol) into anhydrous toluene, heat and stir the resulting mixture and then reflux, to which Put [bis(tri-tert-butylphosphine)]palladium (0.39mmol, 0.02eq). After cooling, it was filtered through a celite / silica gel funnel, and the filtrate was distilled under reduced pressure to remove the organic solvent, and then recrystallized to prepare a pure product of the compound. The relevant data of the obtained target compounds are shown in Table 2.
[0074] Taking compound 1 as an example to illustrate the specific details of the synthesis example: under a nitrogen atmosphere, the raw material c-A-1 (8.02g, 19.55mmol), intermediate 1 (10.54g, 20.14mmol) and sodium tert-butoxide (2.6g, 27.0 mmol) was put into anhydrous toluene, the resulting mixture was heated and stirred and then ref...
Embodiment 3
[0079] The concrete preparation method of compound is as follows:
[0080] Under a nitrogen atmosphere, put raw material c-B (20mmol), intermediate (I) (21mmol) and sodium tert-butoxide (27.0mmol) into anhydrous toluene, heat and stir the resulting mixture and then reflux, and put [Bis(tri-tert-butylphosphine)]palladium (0.4mmol, 0.02eq). After cooling, it was filtered through a celite / silica gel funnel, and the filtrate was distilled under reduced pressure to remove the organic solvent, and then recrystallized to prepare a pure product of the compound. The relevant data of the obtained target compounds are shown in Table 3.
[0081] Taking compound 37 as an example to illustrate the specific details of the synthesis example: under nitrogen atmosphere, the raw material c-B-37 (8.2g, 20mmol), intermediate 1 (11.0g, 21mmol) and sodium tert-butoxide (2.6g, 27.0mmol) Putting in anhydrous toluene, the resulting mixture was heated and stirred and then refluxed, and [bis(tri-tert-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com