Diphyllin heterocyclic derivative as well as preparation method and application thereof
A technology of basil and its derivatives, applied in the fields of medicinal chemistry and pharmacology, can solve the problems of poor stability of glycosidic bond metabolism, complex chemical synthesis, inactivation of glycosidase hydrolysis, etc., and achieve strong tumor cell proliferation inhibitory activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Dissolve 114mg (0.3mmol) of kaempferol in 3mL of DMF, add 282mg (1.5mmol) of 1,2-dibromoethane and 248mg (1.8mmol) of anhydrous potassium carbonate, and react at room temperature for 6h. TLC detected the end of the reaction, added 30mL of ethyl acetate to dilute, then washed with water and saturated brine successively, MgSO 4 Drying, drying under reduced pressure, column chromatography (prtroleum ether:EtOAc=3:1, R f =0.3) to obtain 100 mg of pale yellow solid. Dissolve the solid in 3ml of DMF, add 200mg (2mmol) of N-methylpiperazine and 552mg (4mmol) of anhydrous potassium carbonate, react at 120°C for 2h, monitor the end of the reaction by TLC, add 20ml of ethyl acetate to dilute , and then washed successively with water, saturated brine, MgSO 4 Drying, drying under reduced pressure, column chromatography (DCM:MeOH=30:1, R f = 0.2) 83 mg of 4-O-[2'-(N-methylpiperazinyl)-ethyl]-sinifolin (2a) was obtained as a pale yellow solid (55% yield in two steps).
[0034] Fo...
Embodiment 2-5
[0036] Example compounds were prepared according to the method of Example 1 above.
[0037] Listed below are the physicochemical data of each compound of 2b-2f:
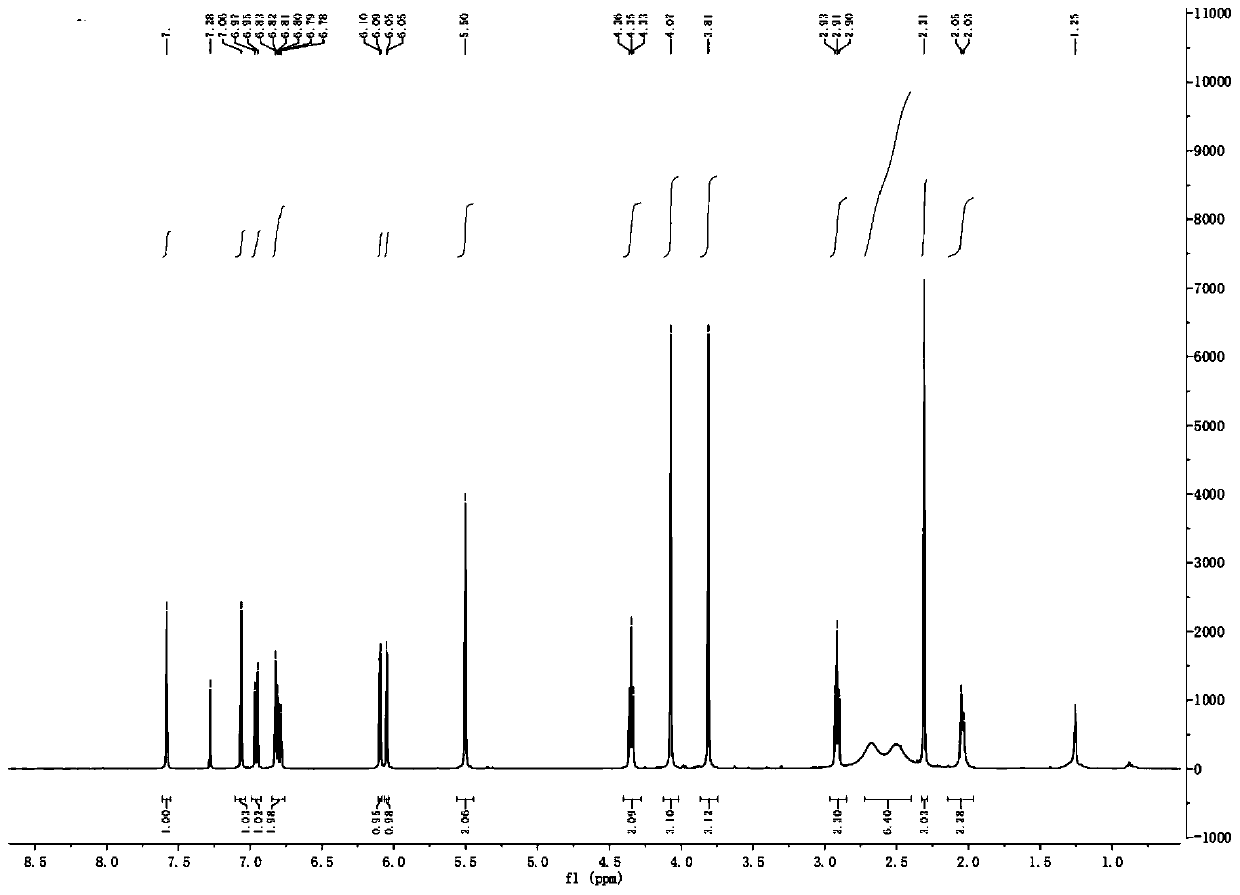
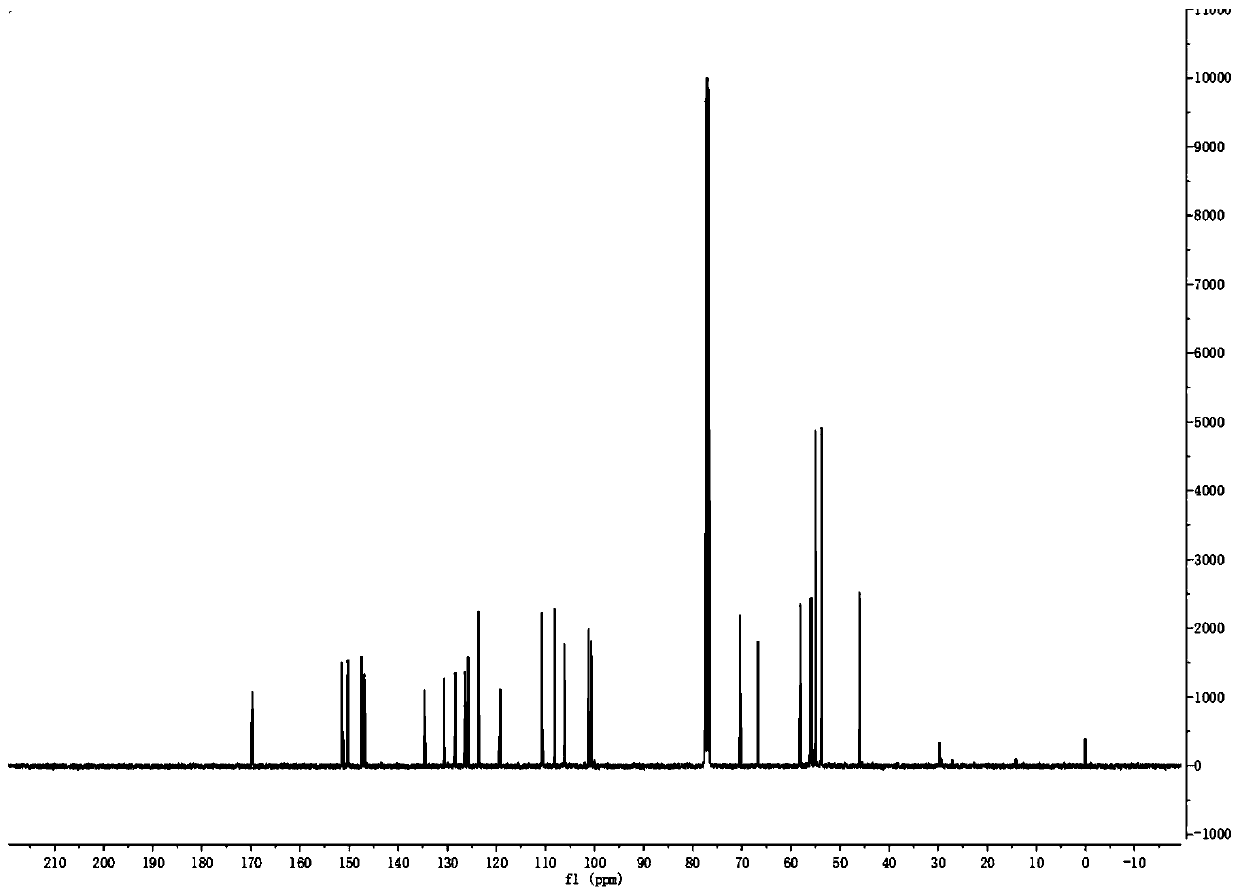
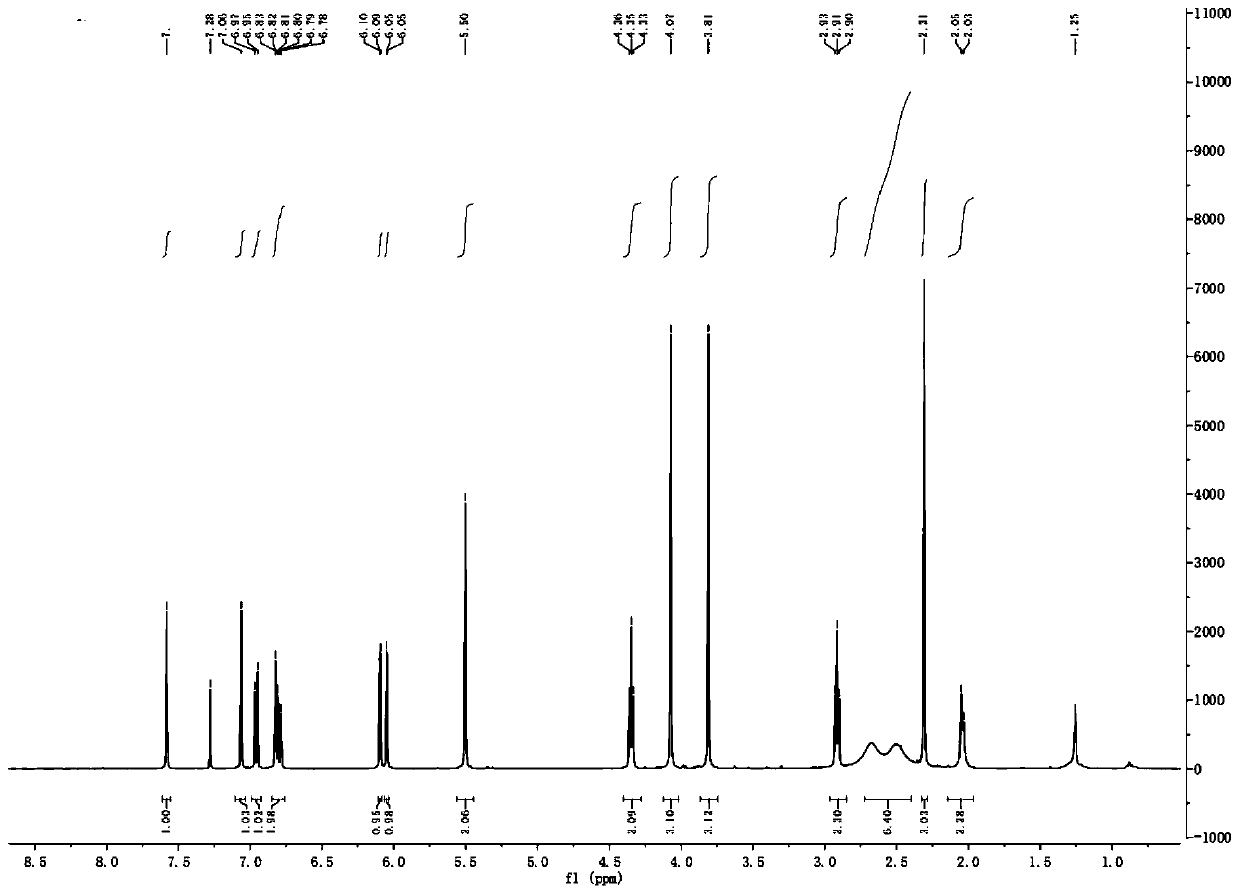
[0038] 2b: 1 H NMR (400MHz, CDCl 3 )δ: 7.58(s,1H,ArH),7.06(s,1H,ArH),6.96(d,J=7.9Hz,1H,ArH),6.80(m,2H,ArH),6.07(dd,J= 18.0, 1.3Hz, 2H, OCH 2 O),5.50(s,2H,ArCH 2 O), 4.33(t, J=5.6Hz, 2H, OCH 2 ),4.07(s,3H,OCH 3 ),3.81(s,3H,OCH 3 ),3.76(m,1H,CH),2.93(m,2H,NCH 2 ), 2.90(t, J=5.7Hz, 2H, NCH 2 ),2.36(m,2H,NCH 2 ),1.95(m,2H,CH 2 ),1.64(m,2H,CH 2 ); 13 C NMR (100MHz, CDCl 3)δ: 169.7, 151.5, 150.2, 147.5, 147.4, 146.9, 134.7, 130.6, 128.4, 126.5, 125.9, 123.6, 119.2, 110.7, 108.2, 106.1, 101.2, 100.6, 75.6, 66.7, 58.0, ,34.4.HRMS(ESI):m / z calcd for C 28 h 3 o 8 N:508.1969; found:508.1966[M+H] + .
[0039] 2c: 1 H NMR (400MHz, Chloroform-d) δ: 7.55(s,1H,ArH),7.06(s,1H,ArH),6.96(d,J=7.9Hz,1H,ArH),6.80(m,2H,ArH) ), 6.07 (d, J=18.0Hz, 2H, OCH 2 O),5.49(s,2H,ArCH 2 O), 4.25(t, J=6.4Hz, 2H, OCH 2 ),4.06(s,3...
experiment example 1
[0044] Drug Experiment Example 1: Test of the Cytotoxic Activity of Compounds 2a-2f and Paclitaxel on Human Esophageal Cancer Cells (TE-13)
[0045] Human esophageal cancer cells TE-13 were cultured with RPMI1640 medium containing 10% fetal bovine serum, 100 U / mL penicillin and 100 U / mL streptomycin. cells in 5×10 per well 3 concentration into a 96-well plate at 37 °C with 5% CO 2 Incubate for 24 hours in a moist air incubator.
[0046] Compounds 2a-2f were dissolved in DMSO to prepare 1×10 -2 mol / L mother solution, dilute the mother solution to the corresponding concentration with complete medium, inoculate the cells in the logarithmic growth phase on a 96-well plate, add different concentrations of compound solutions after 24 hours of attachment, set 4 parallel wells for each concentration, and culture for 68 hours Then add tetramethyl azolium salt (MTT) solution, continue to cultivate for 4 hours, discard the culture medium, add 150 μL of dimethyl sulfoxide, shake for 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com