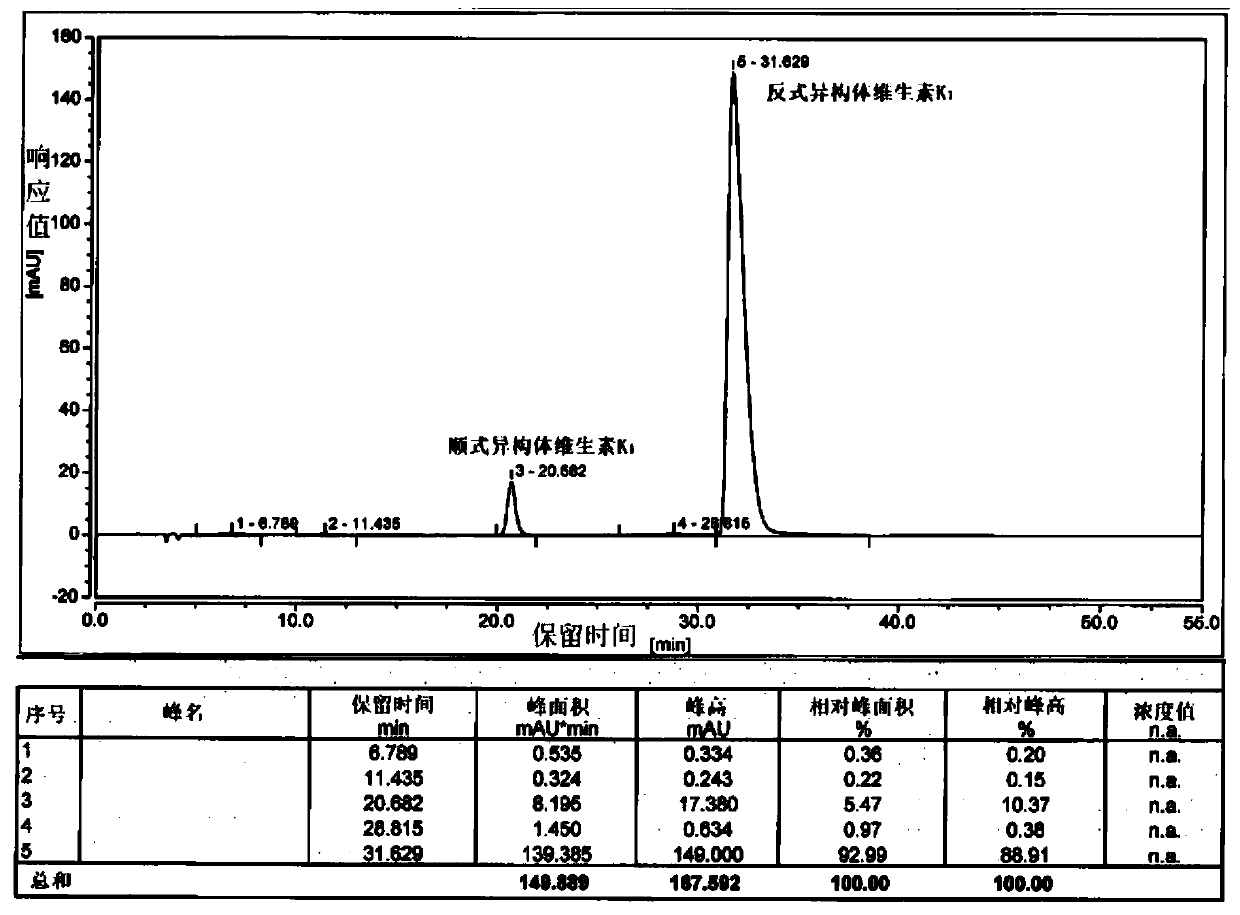
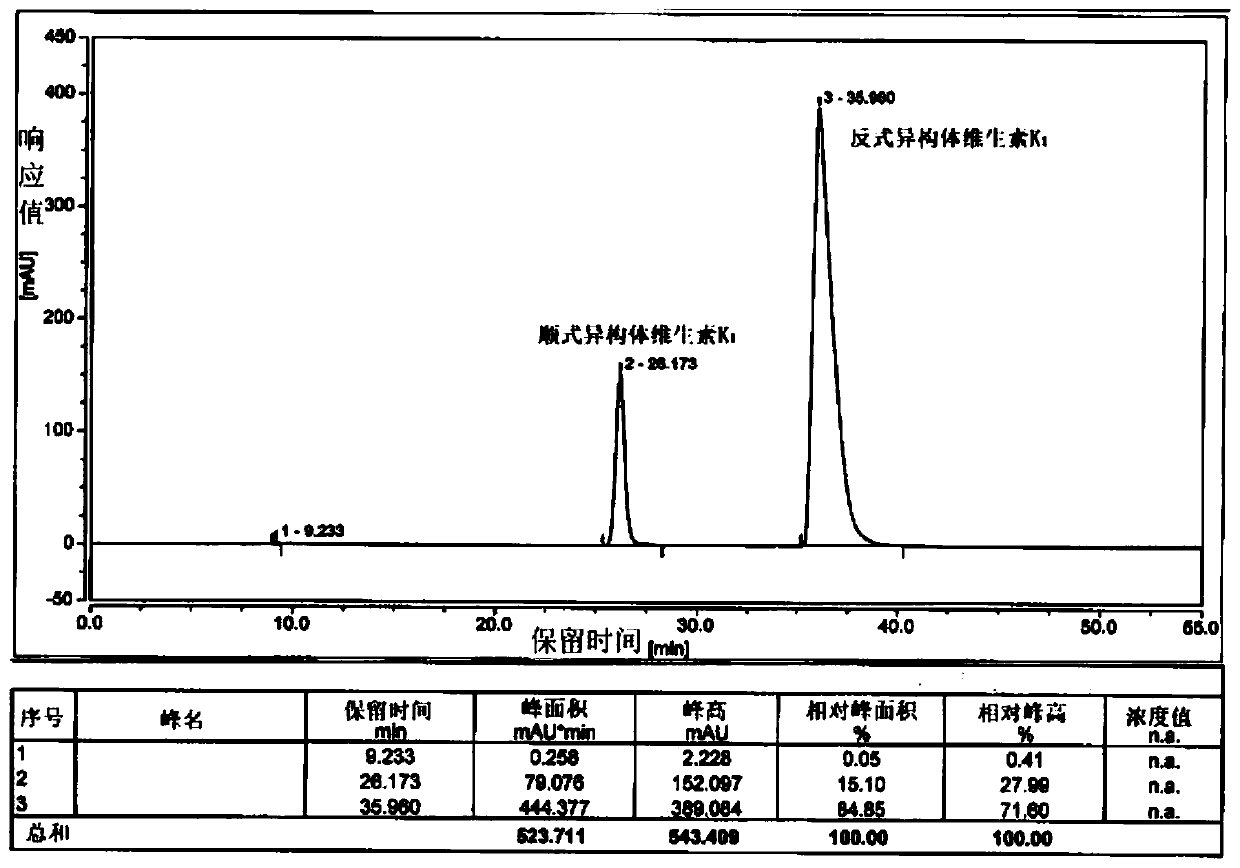
Preparation method of vitamin K1 with different cis-trans isomer proportions and intermediate halogenated phytol thereof
A technology of cis-trans isomerization and phytoalcohol, which is applied in the preparation of halogenated hydrocarbons, organic compounds, quinones, etc. It can solve the problem of uncontrollable isomer ratio and achieve the effect of simple operation and low purification difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]Add 200g of phytoalcohol and 10g of phosphoric acid into 1.2L of n-hexane and stir, cool down to -10-0°C, add 240g of phosphorus tribromide dropwise, and keep the internal temperature under control not higher than 0°C. After 3 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding methanol, separated into layers, spin-dried to remove the solvent, and bromophytol was obtained.
[0042] Add 11.5g of potassium tert-butoxide and 1.2L of tetrahydrofuran into the reaction flask and stir, cool down to -20~-10°C, add 60g of cyclopentadienylmenadione, and slowly add bromophytol dropwise. After 2 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding hydrochloric acid, and the tetrahydrofuran was removed by rotary evaporation. The remaining aqueous phase was extracted with 0.5 L of methyl tert-butyl ether, separated, and the solvent was removed by rotary evaporation to obtain an oily substance. Dissolv...
Embodiment 2
[0044] Add 200g of phytoalcohol and 40g of phosphoric acid into 1.2L of n-hexane and stir, cool down to -10-0°C, add 240g of phosphorus tribromide dropwise, and control the internal temperature to not higher than 0°C. After 2.5 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding methanol, separated into layers, spin-dried to remove the solvent, and bromophytol was obtained.
[0045] Add 11.5g of potassium tert-butoxide and 1.2L of tetrahydrofuran into the reaction flask and stir, cool down to -20~-10°C, add 60g of cyclopentadienylmenadione, and slowly add bromophytol dropwise. After 2 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding hydrochloric acid, and the tetrahydrofuran was removed by rotary evaporation. The remaining aqueous phase was extracted with 0.5 L of methyl tert-butyl ether, separated, and the solvent was removed by rotary evaporation to obtain an oily substance. Dissolve the...
Embodiment 3
[0047] Add 200g of phytoalcohol and 60g of phosphoric acid into 1.2L of n-hexane and stir, lower the temperature to -10-0°C, add 240g of phosphorus tribromide dropwise, and keep the internal temperature under control not higher than 0°C. After 2 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding methanol, separated into layers, spin-dried to remove the solvent, and bromophytol was obtained.
[0048] Add 11.5g of potassium tert-butoxide and 1.2L of tetrahydrofuran into the reaction flask and stir, cool down to -20~-10°C, add 60g of cyclopentadienylmenadione, and slowly add bromophytol dropwise. After 2 hours of monitoring by TLC, the reaction of the raw materials was complete, quenched by adding hydrochloric acid, and the tetrahydrofuran was removed by rotary evaporation. The remaining aqueous phase was extracted with 0.5 L of methyl tert-butyl ether, separated, and the solvent was removed by rotary evaporation to obtain an oily substa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com