Nickel coordination polymer material with wide-temperature-range reversible thermochromic property and preparation method and application of nickel coordination polymer material
A technology of thermochromic materials and coordination polymers, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, thermometers, etc., can solve the problems of small color change and color difference, time-consuming recoloring process, poor material stability, etc., and achieve steps The effect of simplicity, obvious color change, and wide span of color change temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
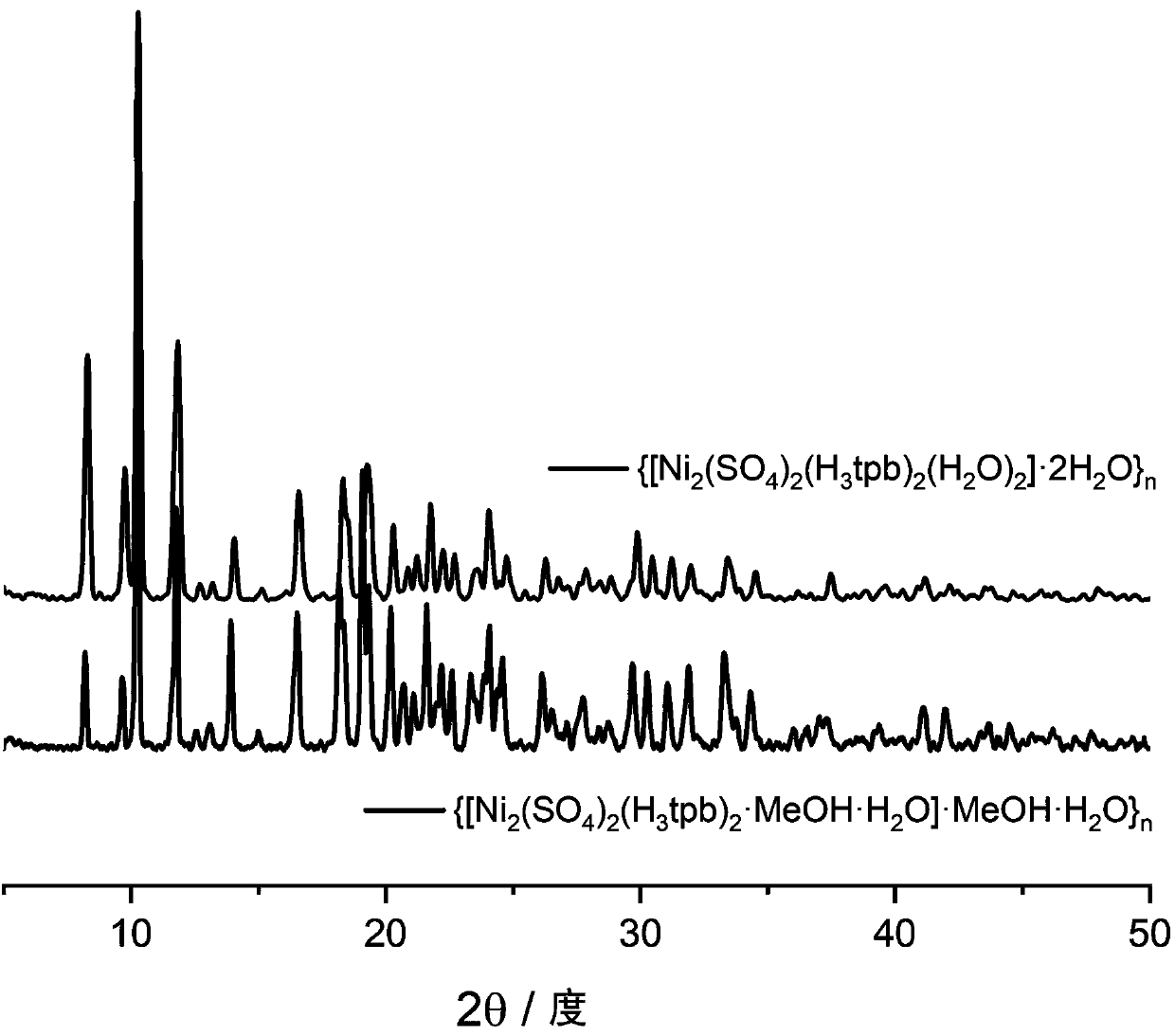
[0027] Embodiment 1: Complex {[Ni 2 (SO 4 ) 2 (H 3 tpb) 2 (H2 O) 2 ]·2H 2 O} n Synthesis
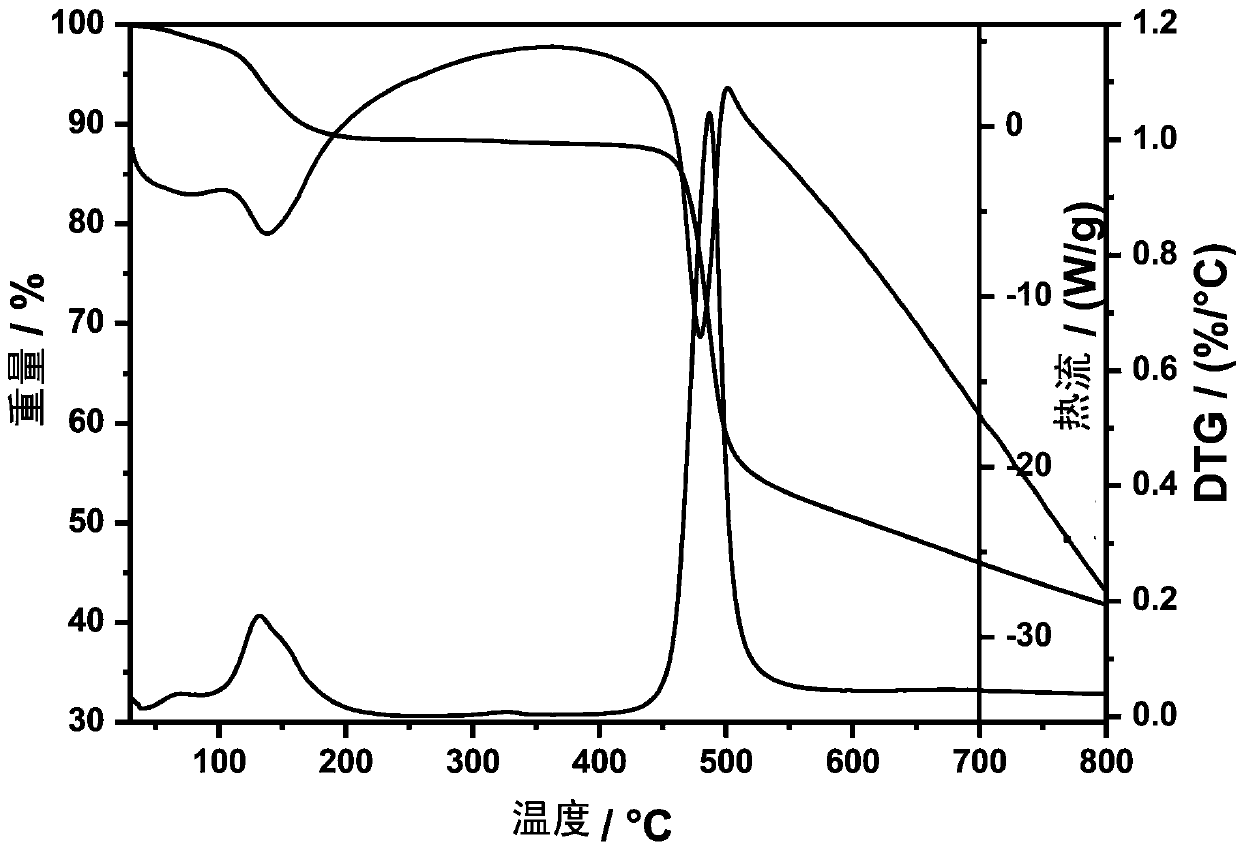
[0028] Add 0.395 g of nickel sulfate heptahydrate and 0.276 g of 1,3,5-(pyrazolyl)benzene into a mixed solution of methanol and water (volume ratio 4:1), and stir with a magnetic stirrer in air at room temperature for 10 minutes , and then transferred to a 25mL hydrothermal reaction kettle with a Teflon substrate and sealed, placed in an oven at 160°C and kept warm for 48 hours, and then cooled at room temperature. Get jade green transparent massive crystal {[Ni 2 (SO 4 ) 2 (H 3 tpb) 2 ·MeOH·H 2 O] MeOH H 2 O} n . Unit cell parameters: a=21.5743(Å), b=21.5743(Å), c=34.449(Å), α=90.000(deg), β=90.000(deg), γ=90.000(deg). Afterwards, the synthesized {[Ni 2 (SO 4 ) 2 (H 3 tpb) 2 ·MeOH·H 2 O] MeOH H 2 O} n At 250°C for 20 hours to remove the solvent molecules methanol and water, after cooling, place in the air to absorb water molecules in the air, and a thermochromic...
Embodiment 2
[0032] Embodiment 2: Complex {[Ni 2 (SO 4 ) 2 (H 3 tpb) 2 (H 2 O) 2 ]·2H 2 O} n Photo of temperature and color change
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com