Preparation method of blue dye based on pyridine/2-aminothiophene/barbituric acid ternary system
A technology of dimethyl barbituric acid and blue dyes, which is applied in the preparation of azo dyes, azo dyes, organic dyes, etc., can solve the problems of small quantity and types of disperse blue dyes, and achieve simple operation steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
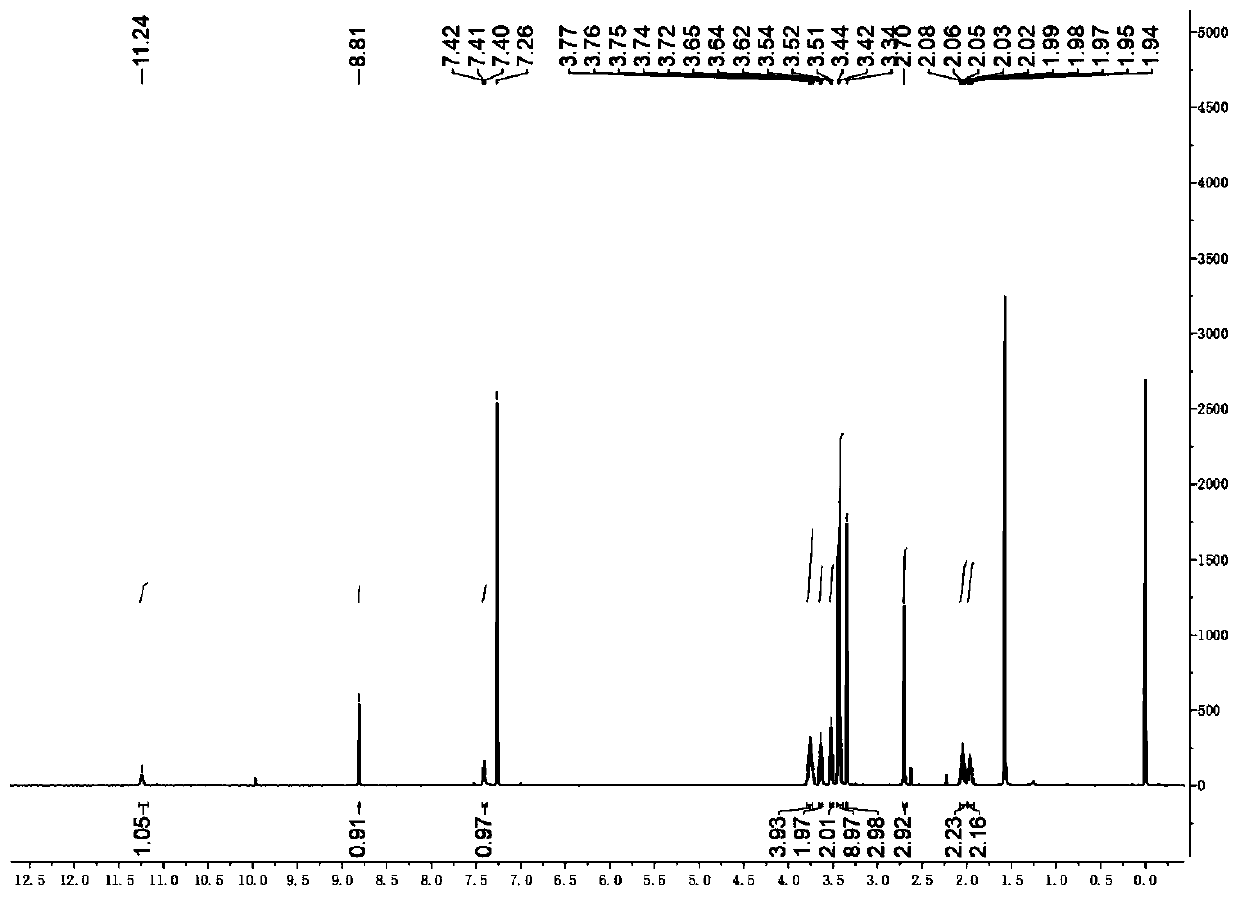
Embodiment 1
[0023] First follow [Zhao XL, Geng J, Huang W. pH-induced azo-keto and azo-enoltautomerism for 6-(3-methoxypropylamino)pyridin-2-one based thiophene azodyes. Dyes Pigm 2017; 147:318-26.] Compound 1 and Compound 2 were prepared by the above process, namely 7 and 13 in Scheme 1, respectively.
[0024] Preparation 1 of compound 3:
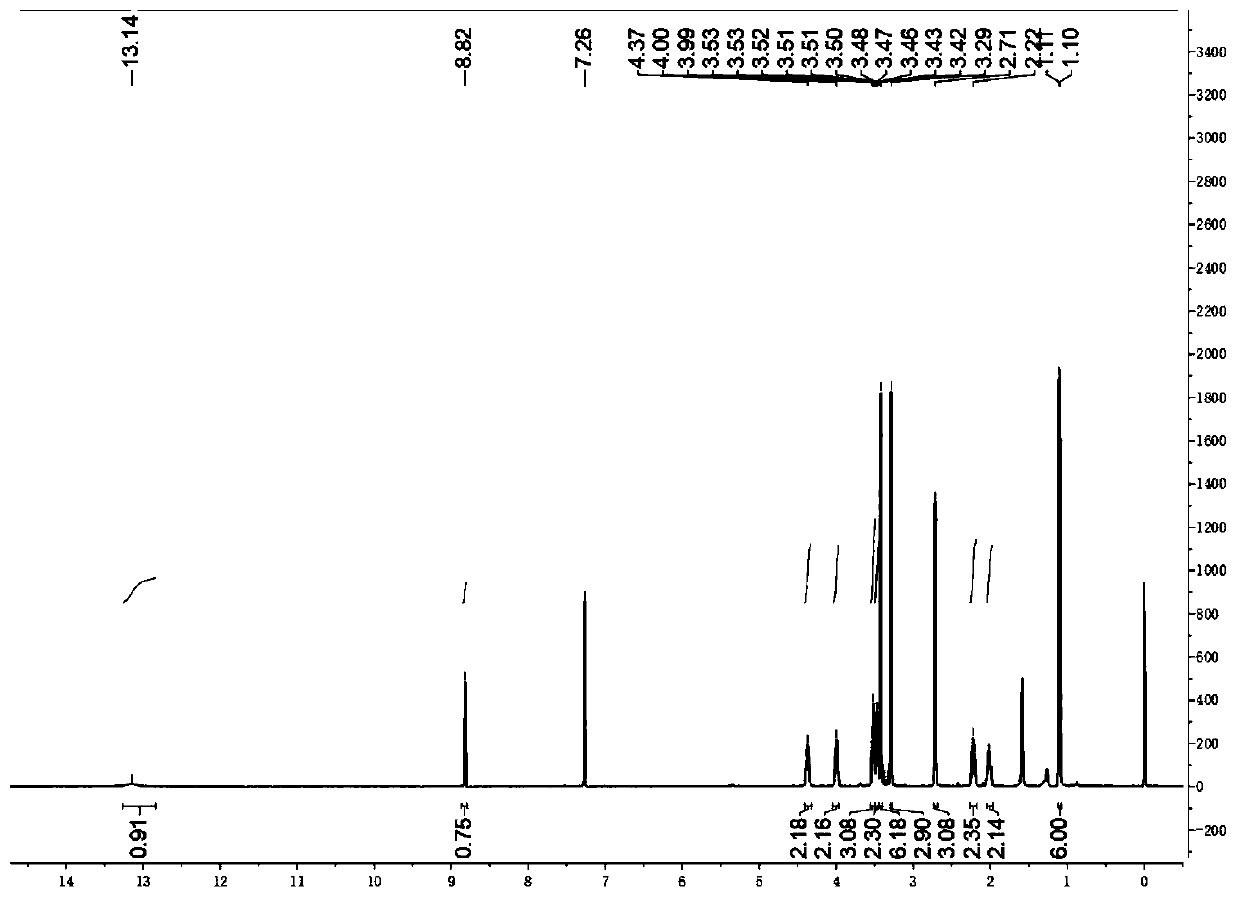
[0025] Weigh 2.59g (5mmol) of compound 1, 1.17g (7.5mmol) of 1,3-dimethylbarbituric acid in an eggplant-shaped bottle, add 5ml of anhydride to fully dissolve it, and reflux at 90°C for 3 hours; After the reaction was monitored by TLC, the heating was stopped, the reaction solution was returned to room temperature, and a solid was precipitated. Then filter under reduced pressure, and wash the filter residue with sufficient water and isopropanol. The crude product was recrystallized from methanol and dichloromethane to obtain compound 3 in 80% yield (2.62 g). 1 H NMR (400MHz, CDCl 3 , ppm): δ=13.14(s, 1H), 8.82(s, 1H), 4.41-4.32(m, 2H), 4.00(m, J=11...
Embodiment 2
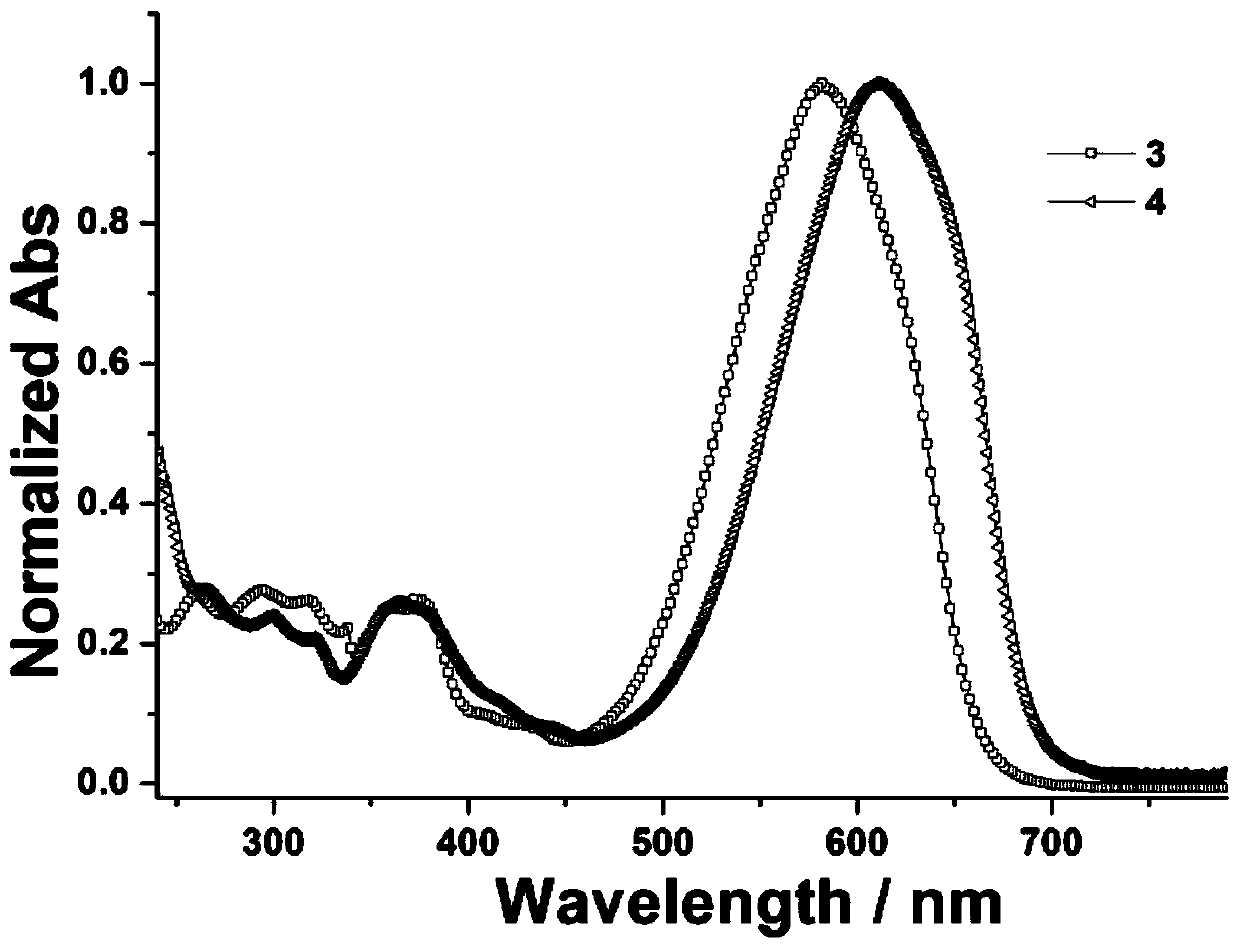
[0032] The concentration is respectively configured as 3.0×10 -5 The dichloromethane solution of mol / L dyestuff 3-4, test its UV-Vis absorption spectrum asfigure 1 shown. The maximum absorption wavelength of the corresponding compound (λ max ), molar extinction coefficient (ε) and width at half maximum (FWHM) are shown in Table 1.
[0033] Table 1 Compounds 1-4 in CH 2 Cl 2 The wavelength of maximum absorption in the solvent (λ max ), molar extinction coefficient (ε) and width at half maximum (FWHM)
[0034]
[0035] It can be seen from the figure that the prepared dye 3 is different from our previously reported reaction precursor dye 1 (derived from [ZhaoXL, Geng J, Huang W. pH-induced azo-keto and azo-enol tautomerism for 6-(3- Compared with methoxypropylamino) pyridin-2-one based thiophene azo dyes. Dyes Pigm 2017; 147:318-26.]), its maximum absorption wavelength is red-shifted from the original 517nm to 582nm. This phenomenon can be interpreted as the introduction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com