Preparation method of tri(2-methyl-2-phenylpropyl)tin m-methyl benzoate complex and application thereof
A technology of toluic acid ester and m-toluic acid, applied in the field of tritin m-toluic acid ester complexes, can solve the problems of high and low anti-cancer activity, application limitation, no anti-cancer activity, etc. The effect of high anticancer activity, simple preparation method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
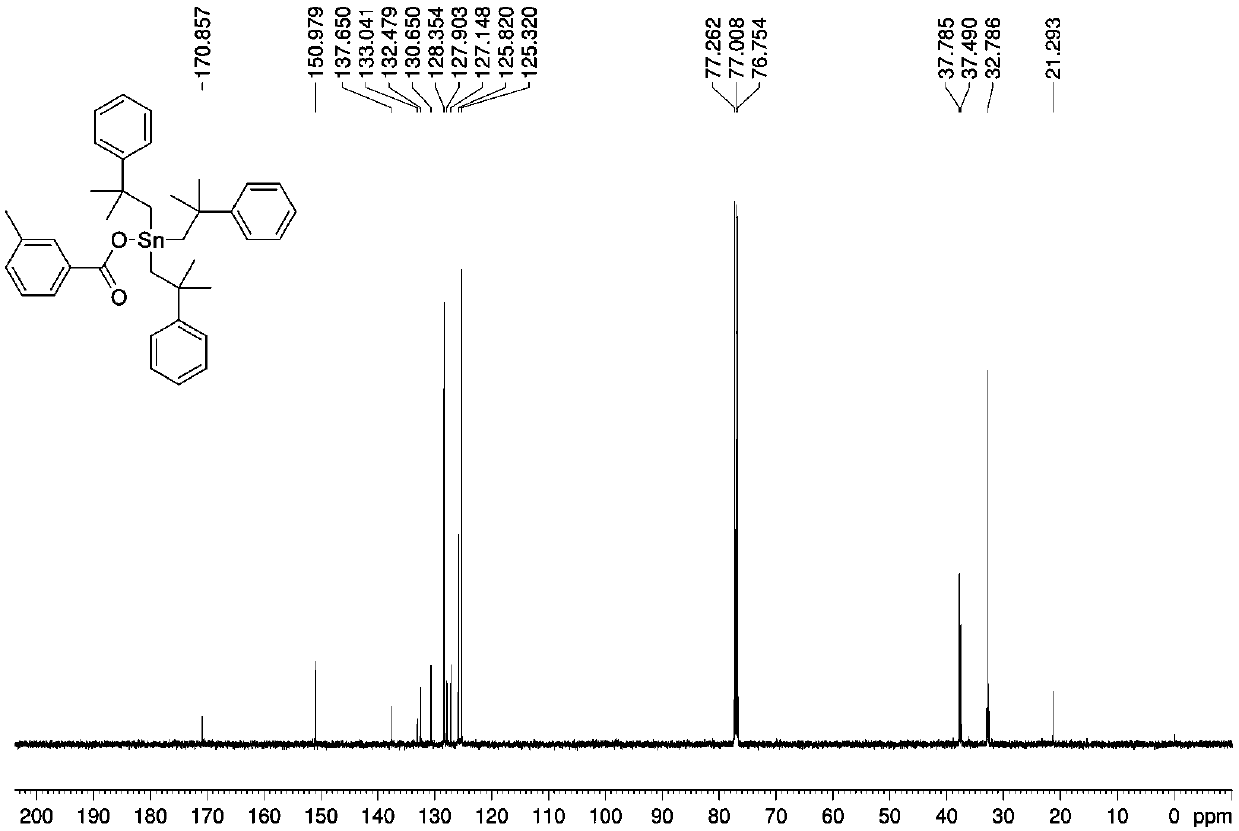
[0032] Preparation of three (2-methyl-2-phenylpropyl) tin m-methylbenzoate complexes:
[0033] Add 1.0532 g (1 mmol) of bis[tris(2-methyl-2-phenylpropyl)]tin oxide, 0.2726 g (2 mmol) of m-toluic acid, and anhydrous methanol into the microwave reaction tank in sequence. 10 mL, under the air atmosphere, the microwave reaction was carried out at a radiation power of 800 W and a temperature of 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilize naturally at room temperature to obtain a yellow oily liquid, which is tris(2-methyl-2-phenylpropyl)tin m-methylbenzoate complex. Yield: 71%.
[0034] Elemental analysis (C 38 h 46 o 2 Sn): theoretical value: C, 69.84; H, 7.10. Found: C, 69.88; H, 7.15.
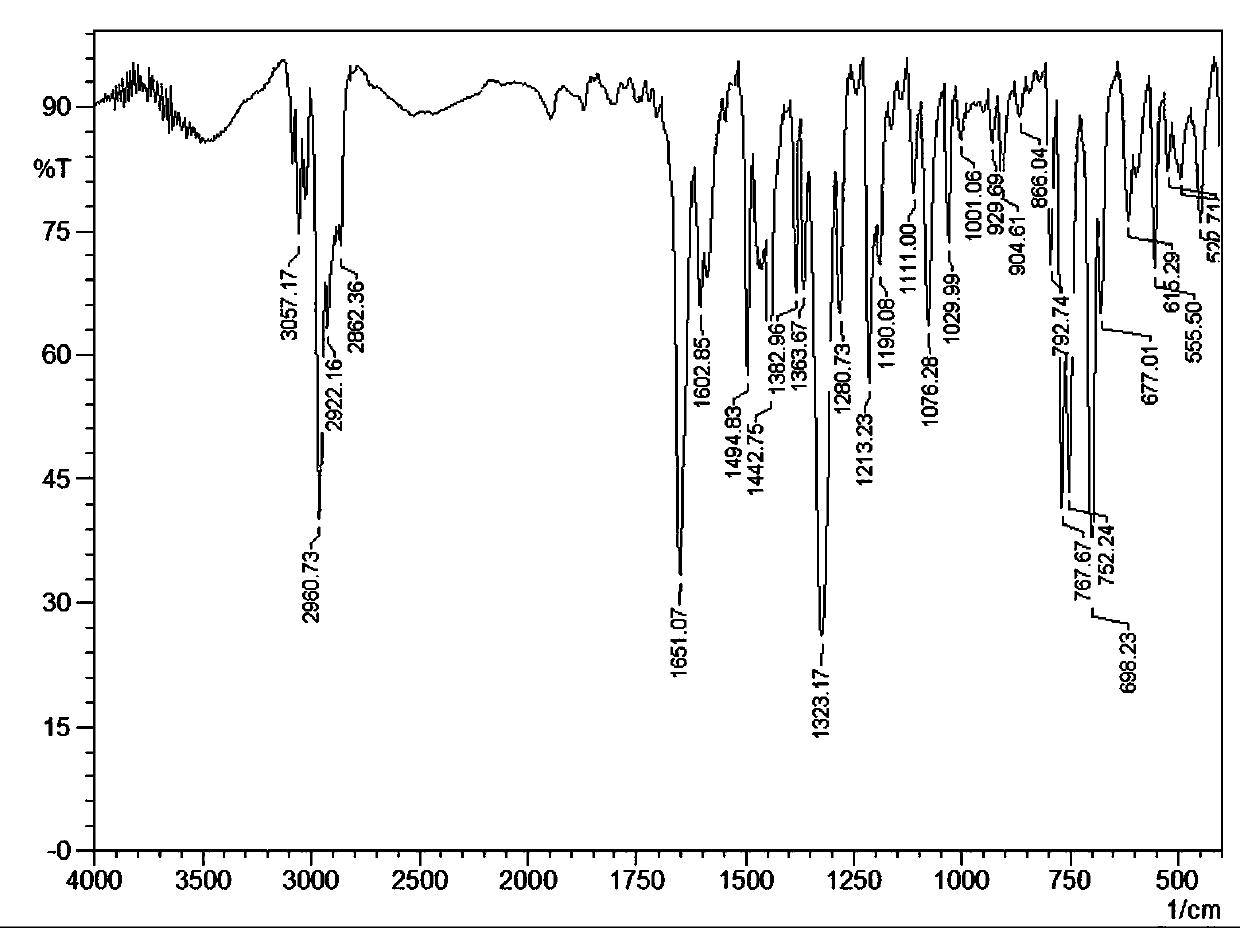
[0035] IR (KBr, v / cm -1): 3057.17 (w), 2960.73 (s), 2922.16 (m), 2862.36 (w), 1651.07 (s), 1602.85 (w), 1494.83 (m), 1442.75 (m), 1382.96 (w), 1363.67 (w ), 1323.17 (s), 1280.73 (w), 1213.23 (m), 1190.08 (w), 1111.00 (w), 1076.28 (m), 1029.99 (...
Embodiment 2
[0040] Preparation of three (2-methyl-2-phenylpropyl) tin m-methylbenzoate complexes:
[0041] Add bis[tris(2-methyl-2-phenylpropyl)]tin oxide 1.0534 g (1.0 mmol), m-toluic acid 0.2865 g (2.1 mmol), solvent anhydrous With 15 mL of methanol, the microwave reaction was carried out under an air atmosphere with a radiation power of 800 W and a temperature of 100 °C for 60 min. After the reaction, cool naturally, filter, and the solvent volatilize naturally at room temperature to obtain a yellow oily liquid, which is tris(2-methyl-2-phenylpropyl)tin m-methylbenzoate complex. Yield: 70%.
[0042] Elemental analysis (C 38 h 46 o 2 Sn): theoretical value: C, 69.84; H, 7.10. Found: C, 69.88; H, 7.15.
[0043] IR (KBr, v / cm -1 ): 3057.17 (w), 2960.73 (s), 2922.16 (m), 2862.36 (w), 1651.07 (s), 1602.85 (w), 1494.83 (m), 1442.75 (m), 1382.96 (w), 1363.67 (w ), 1323.17 (s), 1280.73 (w), 1213.23 (m), 1190.08 (w), 1111.00 (w), 1076.28 (m), 1029.99 (w), 1001.06 (w), 929.69 (w), 904.61...
Embodiment 3
[0048] Preparation of three (2-methyl-2-phenylpropyl) tin m-methylbenzoate complexes:
[0049] Add 1.0537 g (1 mmol) of bis[tris(2-methyl-2-phenylpropyl)]tin oxide, 0.2728 g (2 mmol) of m-toluic acid, and anhydrous methanol into the microwave reaction tank in sequence. 12 mL, under the air atmosphere, the microwave reaction was carried out at a radiation power of 800 W and a temperature of 100 °C for 120 min. After the reaction, cool naturally, filter, and the solvent volatilize naturally at room temperature to obtain a yellow oily liquid, which is tris(2-methyl-2-phenylpropyl)tin m-methylbenzoate complex. Yield: 69%.
[0050] Elemental analysis (C 38 h 46 o 2 Sn): theoretical value: C, 69.84; H, 7.10. Found: C, 69.88; H, 7.15.
[0051] IR (KBr, v / cm -1 ): 3057.17 (w), 2960.73 (s), 2922.16 (m), 2862.36 (w), 1651.07 (s), 1602.85 (w), 1494.83 (m), 1442.75 (m), 1382.96 (w), 1363.67 (w ), 1323.17 (s), 1280.73 (w), 1213.23 (m), 1190.08 (w), 1111.00 (w), 1076.28 (m), 1029.99...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com