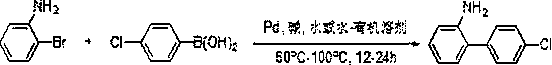Novel synthesis method of 4'-chloro-2-aminobiphenyl
A technology of aminobiphenyl and a new method, which is applied in the preparation of amino compounds from amines, organic chemistry, etc., and achieves the effect of simple treatment and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Put o-bromoaniline (1 mmol), p-chlorophenylboronic acid (1 mmol), sodium carbonate (1 mmol), palladium chloride (1 mol%) in a 25 mL round bottom flask, add water / ethanol (volume ratio 1: 1) Mixed solution 6 mL, 60 o C for 12 h. After the reaction was completed, it was cooled to room temperature, and then extracted with ethyl acetate. After spin-drying the organic solvent, the product was obtained by separation, and the yield was 60%.
Embodiment 2
[0032] Put o-bromoaniline (1 mmol), p-chlorophenylboronic acid (2 mmol), sodium carbonate (3 mmol), palladium acetate (2 mol%) in a 25 mL round bottom flask, add water / ethanol (volume ratio 1:1 ) mixed solution 6 mL, 100 o C under stirring for 24 h. After the reaction, it was cooled to room temperature, and then extracted with ether. After spin-drying the organic solvent, the product was obtained by separation with a yield of 97%.
Embodiment 3
[0034] Put o-bromoaniline (1 mmol), p-chlorophenylboronic acid (1.5 mmol), sodium carbonate (2 mmol), palladium acetate (1 mol%) in a 25 mL round bottom flask, add water / ethanol (volume ratio 1: 1) Mixed solution 6 mL, 80 o C under stirring for 24 h. After the reaction, it was cooled to room temperature, and then extracted with ether. After spin-drying the organic solvent, the product was obtained by separation with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com