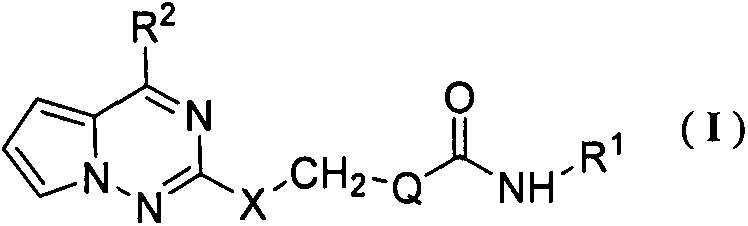
Pyrrolotriazine compounds and applications thereof
A compound, pyrrole technology, applied in the direction of active ingredients of heterocyclic compounds, drug combination, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] N-(2-aminophenyl)-4-[[[4-[(5-methyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4 ]triazin-2-yl]thio]methyl]benzamide (I-1)
[0096]
[0097] Step a: Preparation of 1-amino-1H-pyrrole-2-carboxylic acid methyl ester (1)
[0098] Preparation of monochloramine ether solution: at -25°C, slowly add ammonium chloride (8.00g, 0.150mol) to ether (100mL), add ammonia water (15mL), at -10°C, within 15min Slowly add sodium hypochlorite solution (100mL) dropwise, continue to stir for 15 minutes after the addition, stop the reaction, let it stand for 3 minutes, quickly separate the organic layer, and dry it with anhydrous magnesium sulfate at -25°C for 1 hour, this is the diethyl ether of monochloramine solution.
[0099] Dissolve methyl 1H-pyrrole-2-carboxylate (1.00g, 7.99mmol) in anhydrous tetrahydrofuran (15mL), react for 15min under nitrogen protection, add anhydrous tetrahydrofuran dissolved in sodium hydrogen (1.20g, 50.00mmol) The suspension (45 mL) was reacted at room te...
Embodiment 2
[0115] N-(2-amino-4-fluorophenyl)-4-[[[4-[(5-methyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1 , 2,4] Triazin-2-yl] sulfur] methyl] benzamide (I-2)
[0116]
[0117] Step a: N-(2-amino-4-fluorophenyl)-4-[[[4-[(5-methyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f ][1,2,4]triazin-2-yl]thio]methyl]benzamide (I-2)
[0118] Add 7a (150mg, 0.39mmol) and TBTU (140mg, 0.43mmol) prepared above into a 25mL eggplant-shaped bottle, add N,N-dimethylformamide (10mL), and then add N,N-diisopropyl Ethylamine (204 mg, 1.58 mmol) was stirred at room temperature for 1 h, then p-fluoro-o-phenylenediamine (55 mg, 0.43 mmol) was added, and stirring was continued at room temperature for 4 h. TLC detected that the reaction was complete. Add water (80mL) to the reaction solution, extract with ethyl acetate (30mL×3), combine the organic layers, wash with saturated brine (50mL) and add anhydrous sodium sulfate to dry, let stand, filter, evaporate the solvent under reduced pressure, silica gel column After sep...
Embodiment 3
[0120] N-(2-aminophenyl)-4-[[[4-[(1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazine-2 -yl]sulfur]methyl]benzamide (I-3)
[0121]
[0122] Step a: 4-[[[4-[(1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]thio]methyl ] Preparation of ethyl benzoate (6b)
[0123] 5 (500 mg, 1.44 mmol) prepared above was dissolved in N,N-dimethylformamide (20 mL), potassium iodide (2.39 g, 14.38 mmol) was added, and stirred at 70° C. for 5 minutes. Then N,N-diisopropylethylamine (929mg, 7.19mmol) and 3-aminopyrazole (239mg, 2.88mmol) were added, reacted at 80°C for 36h, and the reaction was complete by TLC detection. Add water (80mL) to the reaction solution, extract with ethyl acetate (30mL×3), combine the organic layers, wash with saturated brine (50mL) and add anhydrous sodium sulfate to dry, let stand, filter, evaporate the solvent under reduced pressure, silica gel column After separation by chromatography (petroleum ether: ethyl acetate = 3:1), 314 mg of white solid was obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com