Hank cetuximab combinations and methods
A composition and antibody technology, applied in chemical instruments and methods, biochemical equipment and methods, drug combinations, etc., can solve the problems that have not yet been used in combination with doxorubicin and immunotherapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example I
[0055] Example I: Immunotherapy of a patient with advanced chordoma
[0056] Therapeutic compositions and modalities used in Example I include various biomolecules and compositions shown in Table 1 below.
[0057]
[0058]
[0059] Table 1
[0060] Treatment was administered in 2 phases, an induction phase and a maintenance phase, as described below. Subjects continued induction therapy for up to 1 year, or until they experienced progressive disease (PD) or unacceptable toxicity (not correctable by dose reduction). Those patients with a complete response (CR) during the induction phase entered the maintenance phase of the study. Subjects may remain in the maintenance phase of the study for a maximum of 1 year. Treatment continued in the maintenance phase until subjects experienced PD or unacceptable toxicity (uncorrectable by dose reduction). The maximum duration of study treatment (including both induction and maintenance phases) was 2 years.
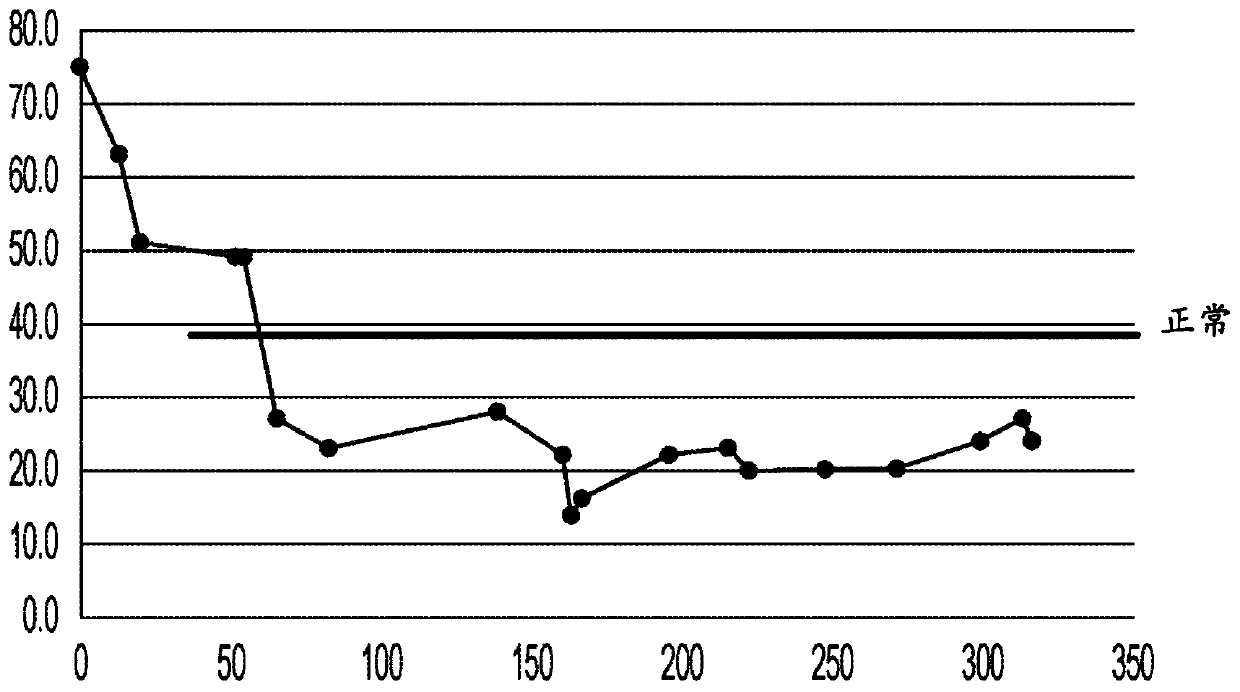
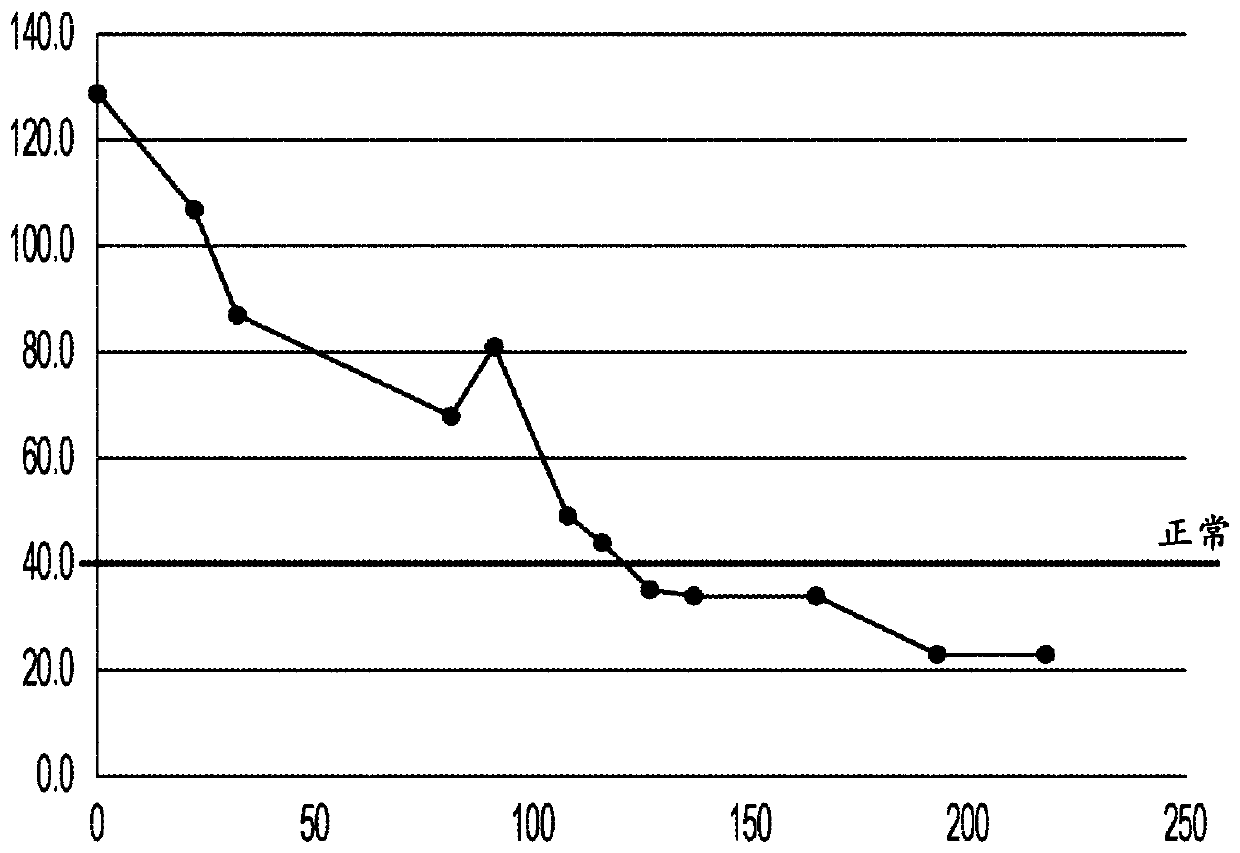
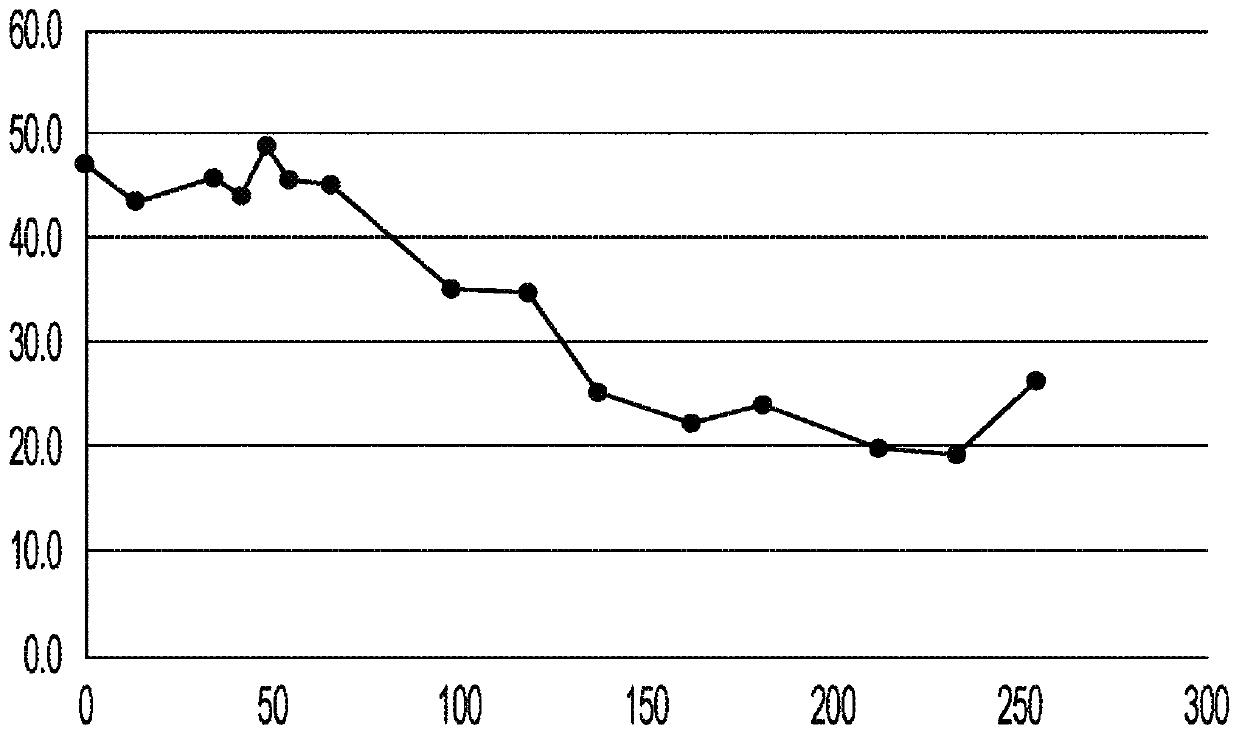
[0061] Tumor biopsie...
example II
[0080] Example II: NANT Squamous Cell Carcinoma (SCC) Vaccines: Molecular Information Integrative Immunotherapy Combining Innate High-Affinity Natural Killer (haNK) Cell Therapy with Adenovirus and Yeast-Based Vaccines T-cell responses in subjects with SCC who progressed on or after cytodeath protein 1 (PD-1) / programmed death-ligand 1 (PD-L1) therapy.
[0081] Therapeutic compositions and modalities used in Example II include various biomolecules and compositions shown in Table 2 below.
[0082]
[0083]
[0084] Table 2
[0085] Treatment in this example is offered to patients who were previously treated with platinum-based chemotherapy and anti-PD-1 / PD-L1 therapy and whose tumors progressed on or after platinum-based chemotherapy and anti-PD-1 / PD-L1 therapy. Treatment in this example was administered in 2 phases, an induction phase and a maintenance phase, as described below. Subjects continued induction therapy for a maximum of 1 year. Those patients with a complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com