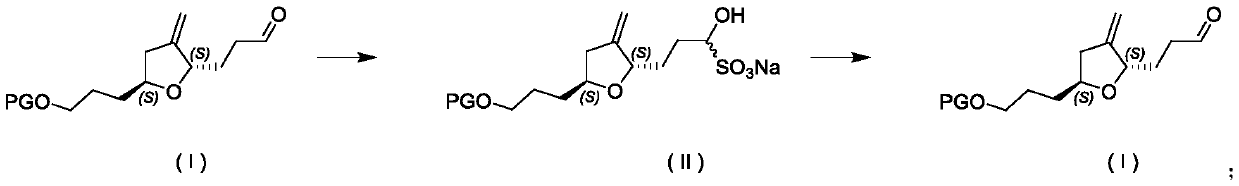
Refining method for improving optical purity of eribulin intermediate compound
A technology of optical purity and purification method, applied in the fields of organic chemistry methods, chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, etc. The product has high optical purity, fast speed, and avoids the effect of column chromatography
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
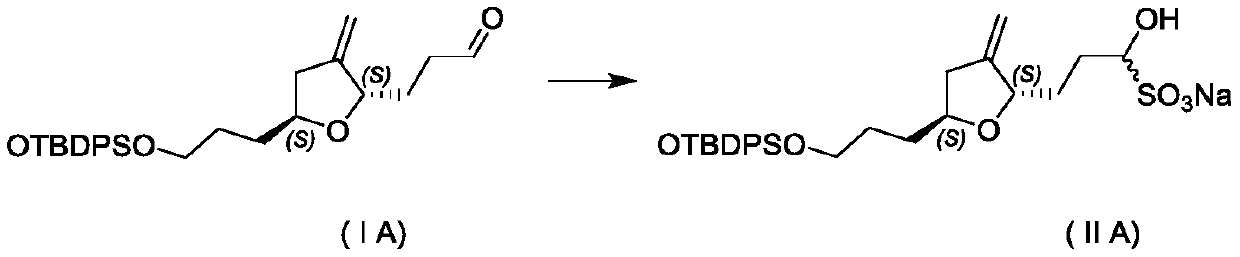
[0028] (1) Preparation of compound formula (ⅡA)
[0029]
[0030] (a) Add 100 grams of compound formula (IA), 500 grams of methanol, 200 grams of water, and 100 grams of sodium bisulfite into a 2L reaction flask, start stirring, and react at 25-30°C;
[0031] (b) After the reaction was complete, filter with suction, wash the filter cake with 20 g of methanol, filter with suction, collect the filter cake, and dry in vacuum at 30° C. to obtain 114.1 g of compound (ⅡA) as a white solid, with a yield of 92.0%.
[0032] (2) Preparation of compound formula (IA)
[0033]
[0034] (c) Add 100 grams of compound formula (ⅡA) and 400 grams of ethyl acetate to the reaction flask respectively, heat up and reflux until the solid dissolves, slowly add 120 grams of n-heptane, continue stirring for 30 minutes after adding, turn off the heating and slowly cool down to Recrystallize at 25°C to precipitate a solid, filter with suction, and collect the filter cake.
[0035] (d) filter cake...
Embodiment 2
[0037] (1) Preparation of compound formula (ⅡA)
[0038]
[0039] (a) Add 100 grams of compound formula (IA), 500 grams of toluene, 200 grams of water, and 100 grams of sodium bisulfite into a 2L reaction bottle, start stirring, and react at 20°C;
[0040] (b) After the reaction is complete, filter with suction, rinse the filter cake with 20 g of tetrahydrofuran, filter with suction, collect the filter cake, and dry in vacuum at 30° C. to obtain 114.1 g of compound (ⅡA) as a white solid, with a yield of 91.0%.
[0041] (2) Preparation of compound formula (IA)
[0042]
[0043] (c) Add 100 grams of compound formula (ⅡA) and 400 grams of water to the reaction flask, raise the temperature and reflux until the solid dissolves, slowly add 120 grams of n-hexane, continue stirring for 30 minutes after adding, turn off the heating and slowly cool down to 20°C A solid crystallized out and was suction filtered to collect the filter cake.
[0044] (d) filter cake, 500 grams of et...
Embodiment 3
[0046] (1) Preparation of compound formula (ⅡA)
[0047]
[0048] (a) Add 100 grams of compound formula (IA), 500 grams of N,N,-dimethylformamide, 200 grams of water, and 100 grams of sodium bisulfite into a 2L reaction bottle, start stirring, and react at 80°C;
[0049] (b) Complete reaction, suction filtration, rinse the filter cake with 20 g of isopropanol, suction filtration, collect the filter cake, and vacuum-dry at 30° C. to obtain 114.1 g of white solid compound (ⅡA), with a yield of 91.8%.
[0050] (2) Preparation of compound formula (IA)
[0051]
[0052] (c) Add 100 grams of compound formula (ⅡA) and 400 grams of xylene to the reaction flask respectively, heat up and reflux until the solid dissolves, slowly add 120 grams of n-pentane, continue stirring for 30 minutes after adding, turn off the heating and slowly cool down to 80 °C recrystallized to precipitate a solid, filtered with suction, and collected the filter cake.
[0053] (d) filter cake, 500 grams ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com