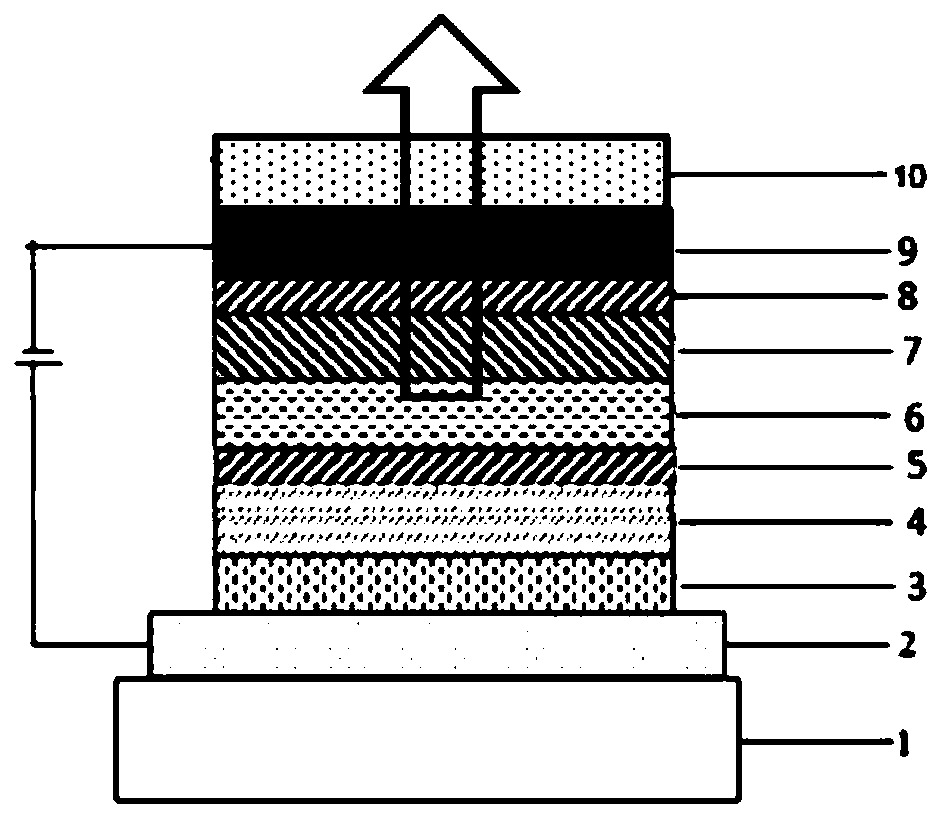
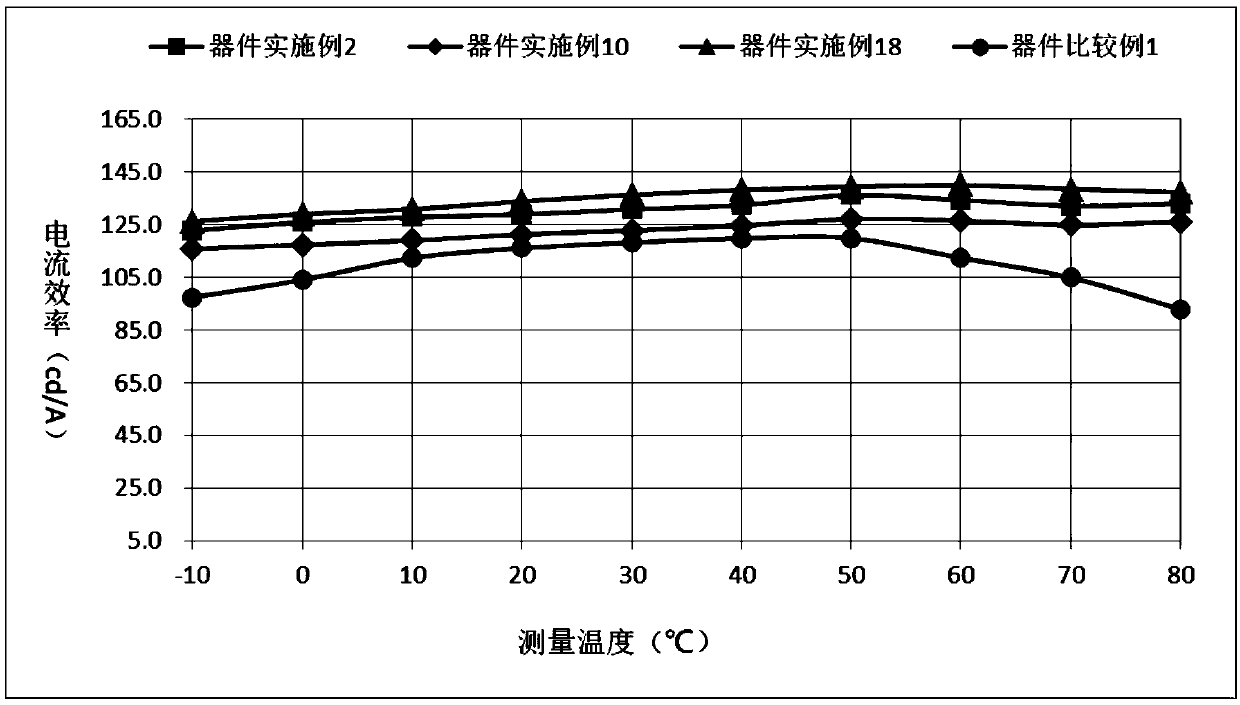
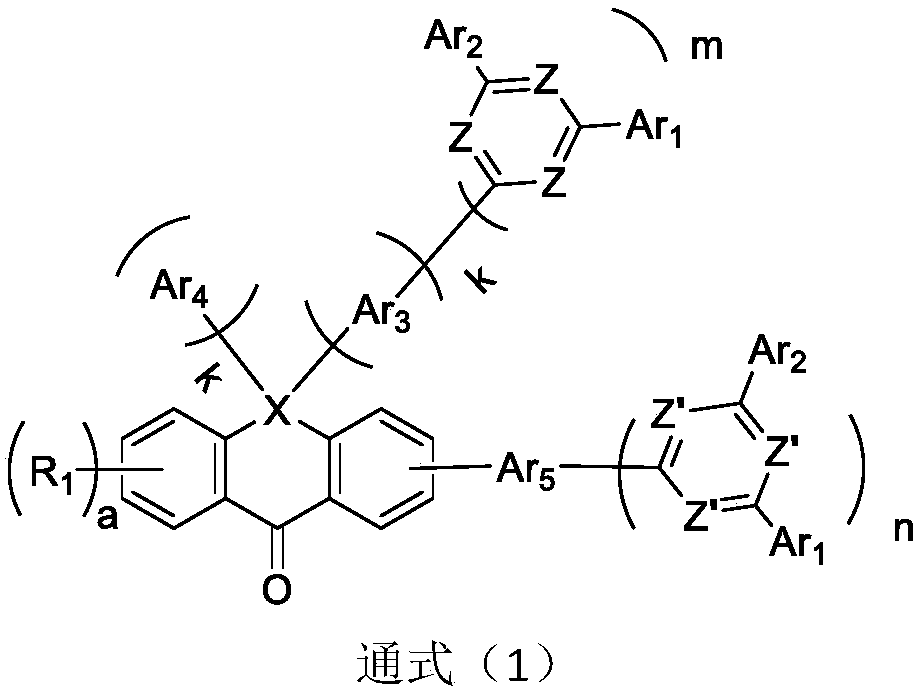
Compound containing anthrone and azacycle and application of compound to OLED
A compound, nitrogen heterocycle technology, applied in the field of compounds containing anthrone and nitrogen heterocycle, can solve different problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The synthesis of embodiment 1 intermediate M:
[0082]
[0083] (1) Under a nitrogen atmosphere, weigh raw material B and dissolve it in tetrahydrofuran, then add raw material C and tetrakis(triphenylphosphine)palladium, stir the mixture, then add potassium carbonate aqueous solution, and mix the above-mentioned reactants at the reaction temperature At 70-90°C, heat to reflux for 5-20 hours. After the reaction, cooling and adding water, the mixture was extracted with dichloromethane, the extract was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure, the obtained residue was purified by silica gel column to obtain intermediate I;
[0084] The molar ratio of raw material B to raw material C is 1:1.0~1.5, the molar ratio of tetrakis(triphenylphosphine) palladium to raw material B is 0.001~0.02:1, and the molar ratio of potassium carbonate to raw material B is 1.0~2.0 :1, the ratio of THF dosage to raw material B is 1g:10~30ml.
[00...
Embodiment 2
[0107] The synthesis of embodiment 2 intermediate S and intermediate T:
[0108] When X=O:
[0109]
[0110] Take the synthesis of intermediate S-1 as an example:
[0111]
[0112] The specific reaction process is:
[0113] (1) In a 500mL three-necked flask equipped with a Dean-Stark separator, feed nitrogen, add 0.1mol of raw material A, 0.2mol of potassium carbonate, 125ml of toluene, and 125ml of DMF, stir, reflux for 4 hours, and dehydrate, Until no more water is produced in the system, about 100 mL of toluene is removed by using a Dean-Stark separator. Naturally cooled to room temperature, 0.12 mol of raw material K was added, and the reaction was refluxed under nitrogen atmosphere for 24 hours. After the reaction, add 200ml of toluene to dilute the system, wash the organic phase with water (200mL water) 3 times with a separatory funnel, separate layers, take the organic phase and dry it with anhydrous sodium sulfate, filter, and the filtrate is rotary evaporated...
Embodiment 3
[0121] The synthesis of embodiment 3 intermediate O and intermediate P:
[0122] When Ar 3 and Ar 4 For methyl:
[0123]
[0124] Its specific reaction process is similar to that of intermediate T, except that raw material U is replaced by raw material V
[0125] When Ar 3 and Ar 4 When it is phenyl:
[0126]
[0127] Take the synthesis of intermediate P-1 as an example:
[0128]
[0129] The specific reaction process is:
[0130] (1) Under a nitrogen atmosphere, first dissolve 0.2 mol of raw material W in 200 ml of tetrahydrofuran, then add the freshly prepared tetrahydrofuran solution of Grignard reagent phenylmagnesium bromide dropwise to the reaction system, and react under reflux for 3 hours. to room temperature. Then the reaction mixture was slowly poured into dilute hydrochloric acid with a mass concentration of 10%, stirred for 15 minutes, separated, the organic phase was collected, dried, and the crude intermediate F obtained by rotary evaporation was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com