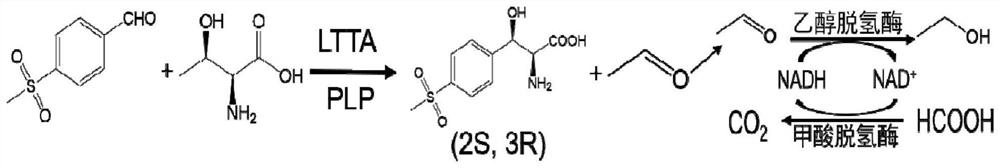
Method for the asymmetric synthesis of (2s,3r)-p-thiamphenylphenylserine in whole cells with "multiple enzymes in one bacterium"
A technology of thiamphenylphenylserine and whole cells, which is applied in the fields of biocatalysis and enzyme engineering, can solve the problems of low yield of target products, lack of short reaction time, cumbersome and time-consuming operations, etc., so as to save the fermentation production and purification preparation process , It is beneficial to industrial production and application, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Preferred embodiments of the present invention are provided below to help further understanding of the present invention. Those skilled in the art should understand that the descriptions of the embodiments of the present invention are only exemplary, and are not intended to limit the solution of the present invention.
[0025] "One bacterium multi-enzyme" method for the asymmetric synthesis of (2S,3R)-p-thiamphenylphenylserine in whole cells, prepared by the following method: Step 1: Construction of double-plasmid engineering bacteria
[0026] L-threonine transaldolase is encoded by the gene PsLTTA, and the recombinant expression plasmid pET28a-PsLTTA is constructed; alcohol dehydrogenase and formate dehydrogenase are encoded by the genes ApADH and CbFDH, respectively, and the recombinant expression plasmid pETDuet-ApADH / CbFDH is constructed; the recombinant Transform Escherichia coli BL21(DE3) with expression plasmids pETDuet-ApADH / CbFDH and pET28a-PsLTTA to construct ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com