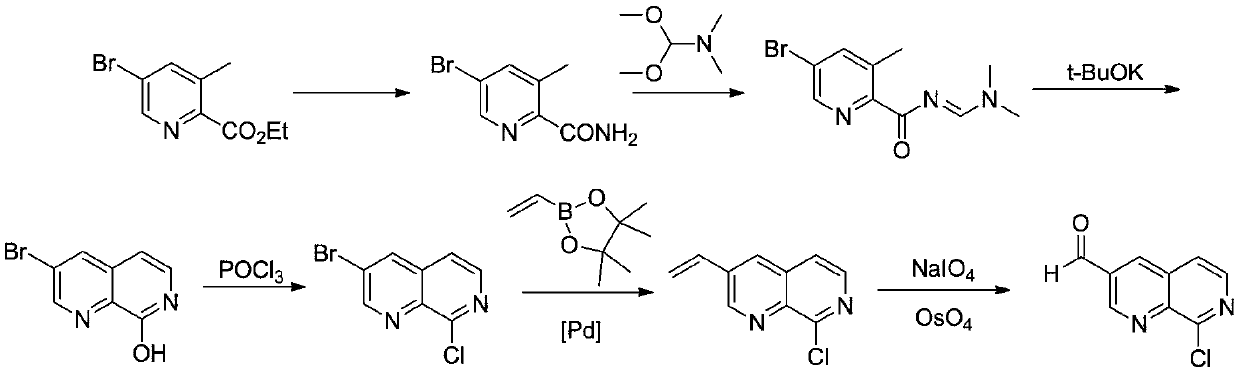
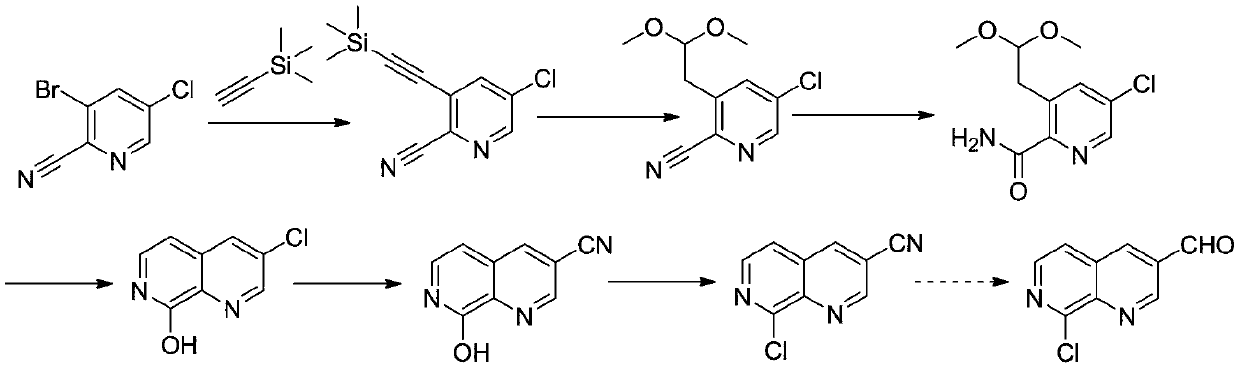
Preparation method of medical intermediate 8-chloro-1, 7-naphthyridin-3-formaldehyde
A synthesis method and intermediate technology are applied in the field of synthesis of pharmaceutical intermediate 8-chloro-1,7-naphthyridine-3-formaldehyde, which can solve the problems of unfavorable large-scale industrial production, high price, environmental pollution and the like, and achieve raw material Inexpensive, good quality, high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
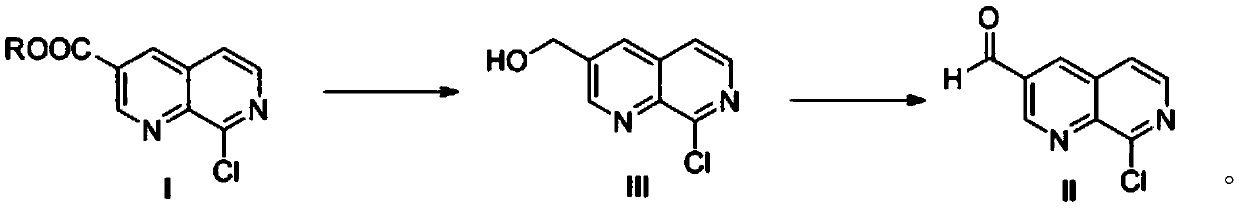
[0031] Step 1) Compound (I) 8-chloro-1,7-naphthyridine-3-carboxylate is dissolved in a solvent as a starting material, and reduced to obtain 2-chloro-1,7-naphthalene under the catalysis of a Lewis acid Pyridine-3-methanol, compound (III);
[0032] Under a nitrogen atmosphere, 50.0g of 8-chloro-1,7-naphthyridine-3-carboxylic acid ethyl ester (0.21mol, 1.0eq) was added to the reaction flask, dissolved in 750g of tetrahydrofuran, and 35.2g of calcium chloride (0.32mol, 1.5eq), lower the temperature to 0-10°C, slowly add 11.9g of sodium borohydride (0.32mol, 1.5eq) in batches, slowly raise the temperature to 40-50°C and keep the reaction. After the reaction is complete, control the reaction temperature below 20°C, slowly add 500g of 1N hydrochloric acid solution, separate layers, take the organic phase, distill to a certain volume, add n-heptane to crystallize, filter, and dry to obtain 32.7g of a light yellow solid (yield 80 %), that is, compound (III) 2-chloro-1,7-naphthyridine...
Embodiment 2
[0036] Step 1) Compound (I) 8-chloro-1,7-naphthyridine-3-carboxylate is dissolved in a solvent as a starting material, and reduced to obtain 2-chloro-1,7-naphthalene under the catalysis of a Lewis acid Pyridine-3-methanol, compound (III);
[0037]Under a nitrogen atmosphere, add 46.8g 8-chloro-1,7-naphthyridine-3-methyl carboxylate (0.21mol, 1.0eq) into the reaction flask, dissolve in 750g methyl tert-butyl ether, add 43.6g chloride Zinc (0.32mol, 1.5eq), cool down to 0-10°C, slowly raise the temperature to 40-50°C and keep warm for reaction with boron trifluoride ether solution (containing 21.7g, 0.32mol, 1.5eq) of boron trifluoride. After the reaction is complete, control the reaction temperature below 20°C, slowly add 500g of 1N hydrochloric acid solution, separate layers, take the organic phase, distill to a certain volume, add n-heptane to crystallize, filter, and dry to obtain 33.5g of a light yellow solid (yield 82 %), that is, compound (III) 2-chloro-1,7-naphthyridine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com