Fluorouracil prodrug with low cytotoxicity and preparation method and application thereof
A fluorouracil and cytotoxic technology, which is applied to fluorouracil prodrugs with low cytotoxicity and the fields of preparation and application thereof, can solve the problems of strong toxic and side effects, hinder DNA synthesis, high cytotoxicity, etc., and achieve the effect of good effect and lower killing rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
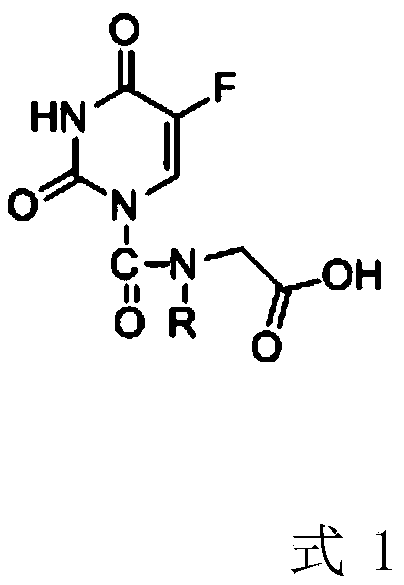
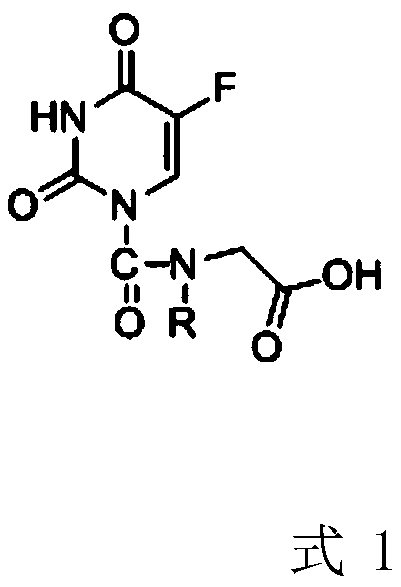
[0022] Example 1 Preparation of 2-(5-fluorouracil-1-carboxamide)acetic acid
[0023] The preparation method of 2-(5-fluorouracil-1-carboxamide) acetic acid is as follows:
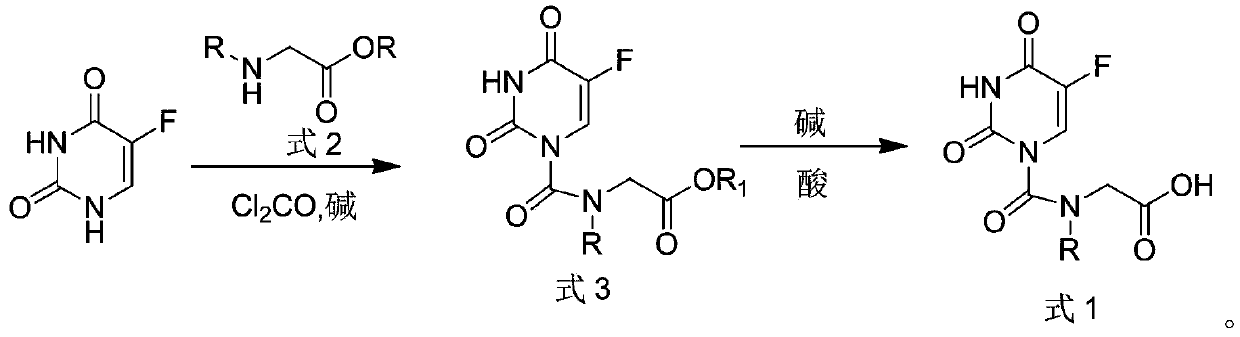
[0024] S1: Add 140 mg of fluorouracil, 118.8 mg of phosgene, 103 mg of glycine ethyl ester shown in formula 2-1 and 20 mL of methanol into the reaction flask, add 136 mg of sodium acetate under stirring conditions, and stir the reaction in an oil bath at 80 °C for 2 h. Monitor After the reaction was completed, methanol was evaporated under reduced pressure, and the concentrate was purified by using petroleum ether / ethyl acetate as the mobile phase with silica gel column to obtain the intermediate product 2-(5-fluorouracil-1-carboxamide) ethyl acetate (formula 3- 1), the yield is 65.4%;
[0025] S2: Add the intermediate product formula 3-1 to 20 mL of 0.2M sodium hydroxide aqueous solution, stir at 10°C, monitor the completion of the reaction, adjust the pH to 7 with 0.2M hydrochloric acid, then add ethanol...
Embodiment 2
[0027] Example 2 Preparation of 2-(N-n-propyl-N-n-propyl-5-fluorouracil-1-carboxamide)acetic acid
[0028] The preparation method of 2-(N-n-propyl-5-fluorouracil-1-carboxamide) acetic acid is as follows:
[0029] S1: Add 140 mg of fluorouracil, 118.8 mg of phosgene, 145 mg of N-n-propylglycine ethyl ester shown in formula 2-2 and 20 mL of methanol into the reaction flask, add 136 mg of sodium acetate under stirring conditions, and put it in an oil bath at 80 °C. The reaction was stirred for 2 h, and after monitoring the completion of the reaction, methanol was evaporated under reduced pressure, and the concentrate was purified by using petroleum ether / ethyl acetate as the mobile phase with silica gel column to obtain the intermediate product 2-(N-n-propyl-5-fluorouracil-1 -Carboxamide) ethyl acetate (formula 3-2), the yield is 62.2%;
[0030] S2: Add the intermediate product formula 3-2 to 20 mL of 0.2M sodium hydroxide aqueous solution, stir at 10°C, monitor the completion o...
Embodiment 3
[0032] Example 3 Preparation of 2-(N-isopropyl-N-isopropyl-5-fluorouracil-1-carboxamide)acetic acid
[0033] The preparation method of 2-(N-isopropyl-5-fluorouracil-1-carboxamide) acetic acid is as follows:
[0034] S1: Add 140 mg of fluorouracil, 118.8 mg of phosgene, 145 mg of N-isopropylglycine ethyl ester shown in formula 2-3 and 20 mL of methanol into the reaction flask, add 136 mg of sodium acetate under stirring conditions, and put it in an oil bath at 80 °C. The reaction was stirred for 2 h, and after monitoring the completion of the reaction, methanol was evaporated under reduced pressure, and the concentrate was purified by using petroleum ether / ethyl acetate as the mobile phase with silica gel column to obtain the intermediate product 2-(N-isopropyl-5-fluorouracil-1 -Carboxamide) ethyl acetate (formula 3-3), yield 62.9%;
[0035] S2: Add the intermediate product formula 3-3 to 20 mL of 0.2M sodium hydroxide aqueous solution, stir at 10°C, monitor the completion of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com