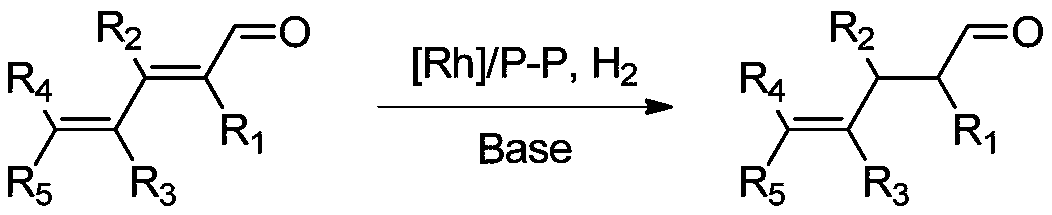
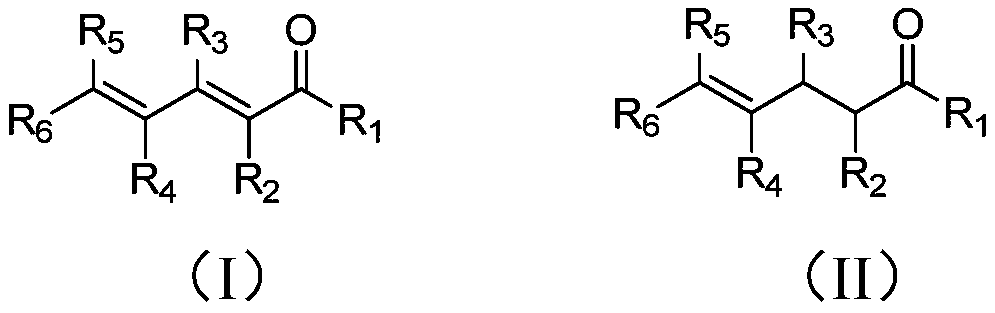Method of preparing gamma-ketene from alpha, gamma-unsaturated diketene
A technology for unsaturated and dienone, applied in the field of preparing γ-ketene, can solve the problems of high selectivity and yield of target product, large amount of catalyst, complicated operation, etc. Small volume and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-18
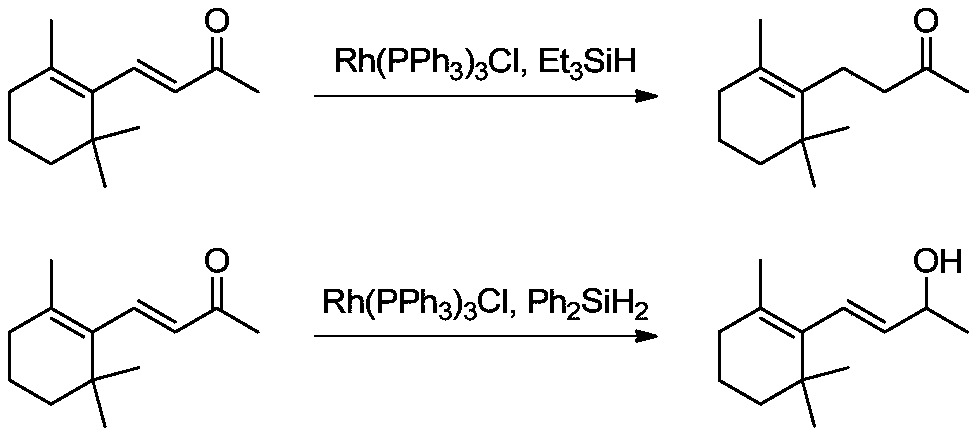
[0039] Selective hydrogenation of β-ionone to dihydro-β-ionone:
[0040] Add tetrakis(triphenylphosphine)palladium (176.87mg, 0.15mmol), triethoxysilane (55.32g, 0.33mol), lanthanum trifluoromethanesulfonate (358.84mg, 0.6mmol) into a 500mL autoclave and tetrahydrofuran (176.60g), the reactor is sealed, and replaced with nitrogen three times, and the nitrogen pressure in the reactor is 0MPa (gauge pressure). Under the protection of nitrogen, pump β-ionone (58.87g, 0.3mol) into the autoclave with an advection pump, turn on the stirring device and heating device of the autoclave, and start timing when the inner temperature of the autoclave reaches 40°C , keep the reaction for 3 hours, take samples for analysis, and detect the conversion rate of β-ionone and the selectivity of dihydro-β-ionone by GC.
[0041] When carrying out embodiment 2-6, monohydrosilane is triethoxysilane, solvent is tetrahydrofuran, and Lewis acid is lanthanum trifluoromethanesulfonate, with respect to embod...
Embodiment 19
[0048] Selective hydrogenation of β-ionone to dihydro-β-ionone:
[0049] Add tetrakis(triphenylphosphine)palladium (35.37mg, 0.03mmol), triethoxysilane (150.8g, 0.9mol), lanthanum trifluoromethanesulfonate (897.11mg, 1.5mmol) into a 1000mL autoclave and tetrahydrofuran (176.60g), the reactor is sealed, and replaced with nitrogen three times, and the nitrogen pressure in the reactor is 0MPa (gauge pressure). Under nitrogen protection, β-ionone (58.87g, 0.3mol) is pumped into the autoclave with a convection pump, and the autoclave stirring device and heating device are turned on. When the inner temperature of the autoclave reaches 60°C, start timing. After incubation and reaction for 6 hours, sample analysis was carried out. GC detection showed that the conversion rate of β-ionone was 98.92%, and the selectivity of dihydro-β-colorone was 98.19%.
Embodiment 20
[0051] Selective hydrogenation of β-ionone to dihydro-β-ionone:
[0052] Add tetrakis(triphenylphosphine)palladium (353.75mg, 0.3mmol), triethoxysilane (50.29g, 0.3mol), lanthanum trifluoromethanesulfonate (179.42mg, 0.3mmol) into a 1000mL autoclave and tetrahydrofuran (294.34g), the reactor was sealed, and replaced three times with nitrogen, and the nitrogen pressure in the reactor was 0MPa (gauge pressure). Under nitrogen protection, β-ionone (58.87g, 0.3mol) was pumped into the autoclave with a convection pump, and the high-pressure autoclave stirring device was turned on. The inner temperature of the autoclave was 20°C, and the temperature was kept for 1 hour, and the sample was analyzed. GC detection showed that the conversion rate of β-ionone was 99.92%, and the selectivity of dihydro-β-ionone was 97.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com