Autosomal dominant Dnajc17 gene mutant as well as application, diagnostic kit and diagnostic gene chip thereof
A diagnostic kit and autosome technology, applied in the field of biomedicine, to achieve the effect of great clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
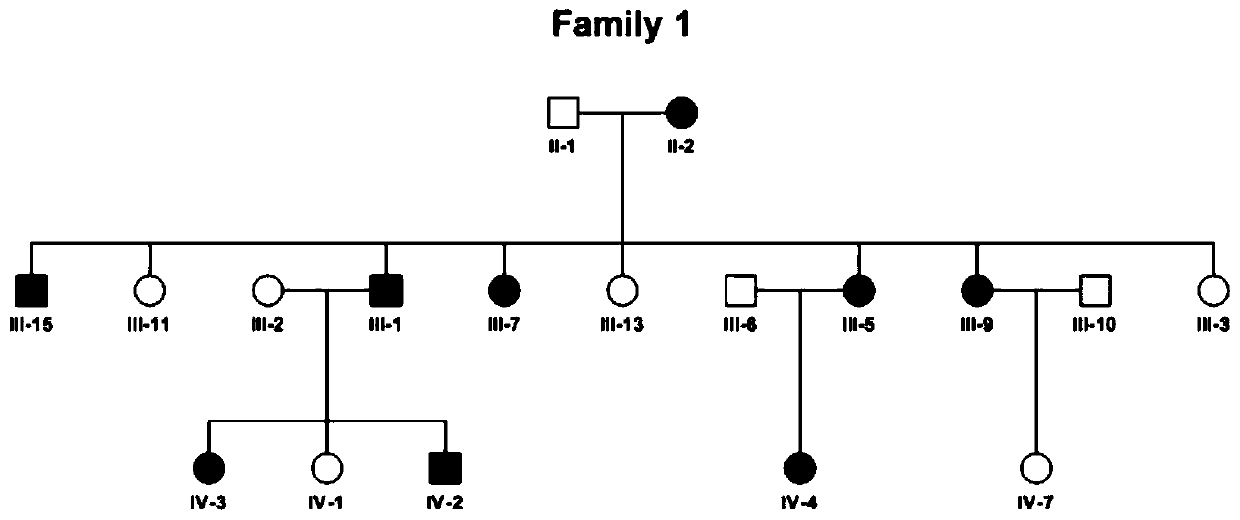
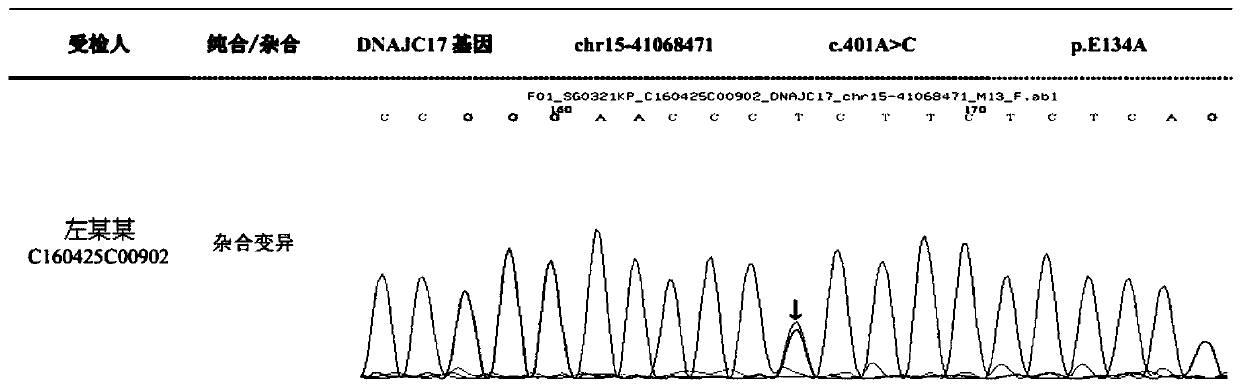
[0035] By performing exome sequencing on a family with autosomal dominant non-syndromic deafness, the present invention found that there is a significant correlation between the mutation of Dnajc17 gene on human chromosome 15 (c. 401A>C, p. E134A) and the occurrence of deafness . Based on this, it was deduced that Dnajc17 could be used as a biomarker for the diagnosis of deafness.
[0036] The verification of the correlation between Dnajc17 and deafness diseases, that is, the determination of Dnajc17 as a biomarker for the diagnosis of deafness: includes the following steps:
[0037] The first step, sample preparation: clinically found a family of acquired deafness (such as figure 1 Shown), in which 9 acquired deafness patients have been diagnosed, the peripheral blood samples of the deafness patients (experimental group, 9 people) and non-deaf individuals (9 people) in the family were extracted respectively.
[0038] The second step, sample exome sequencing and disease-caus...
Embodiment 2
[0043] Application of an autosomal dominant Dnajc17 gene mutant gene mutant in the preparation of a deafness diagnostic kit.
[0044] The nucleotide sequence of the autosomal dominant Dnajc17 mutant is shown in SEQ ID: NO: 2, and its specific PCR upstream and downstream primers are designed by Primer 5, and Invitrogen Company is responsible for primer synthesis. The purity is PAGE grade. The synthesized primers Use RNase free H2O to dissolve, the total concentration is 10 μM.
[0045] A diagnostic kit for an autosomal dominant Dnajc17 gene mutant comprising:
[0046] (a) DNA extraction system:
[0047] QIAmp Blood Kit;
[0048] (b) PCR system:
[0049] PrimeSTAR HS DNA Polymerase 50μL;
[0050] buffer 100μL;
[0051] dNTP Mixture (2.5mM each) 250μL;
[0052] Dnajc17 gene mutant specific amplification upstream primer (SEQ ID NO:3), 1 tube, 10 μM, 100 μL / tube;
[0053] Dnajc17 gene mutant specific amplification downstream primer (SEQ ID NO:4), 1 tube, 10 μM, 100 μL / tube; ...
Embodiment 3
[0056] Sequence Detection of Dnajc17 Gene in Peripheral Blood Cells of Deaf Patients
[0057] Genomic DNA samples from the peripheral blood of deaf patients were extracted using the QIAmp Blood Kit to ensure that the 260 / 280 ratio was above 1.8, and stored at -20°C.
[0058] The expression sequence of Dnajc17 was amplified by PCR, and the reaction system was as follows:
[0059] 5×PrimeSTAR Buffer (Mg 2+ Plus) 10 μl
[0060] dNTP mixture (2.5 mM each) 4 μl
[0061] Dnajc17 primer1 (10 μM) 1 μl
[0062] Dnajc17 primer2 (10 μM) 1 μl
[0063] gDNA template (100ng / μl) 1 μl
[0064] PrimeSTAR HS DNA Polymerase 0.5 μl
[0065] f 2 O 32.5 μl
[0066] PCR conditions: 95°C for 5min, (95°C for 30s; 55°C for 30s; 72°C for 2 min, 40 cycles), 72°C for 5min.
[0067] Sequence determination and result analysis:
[0068] Send the PCR reaction product of Dnajc17 to the biological company for sequence determination, and compare the obtained sequence with the reference sequence to find...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com