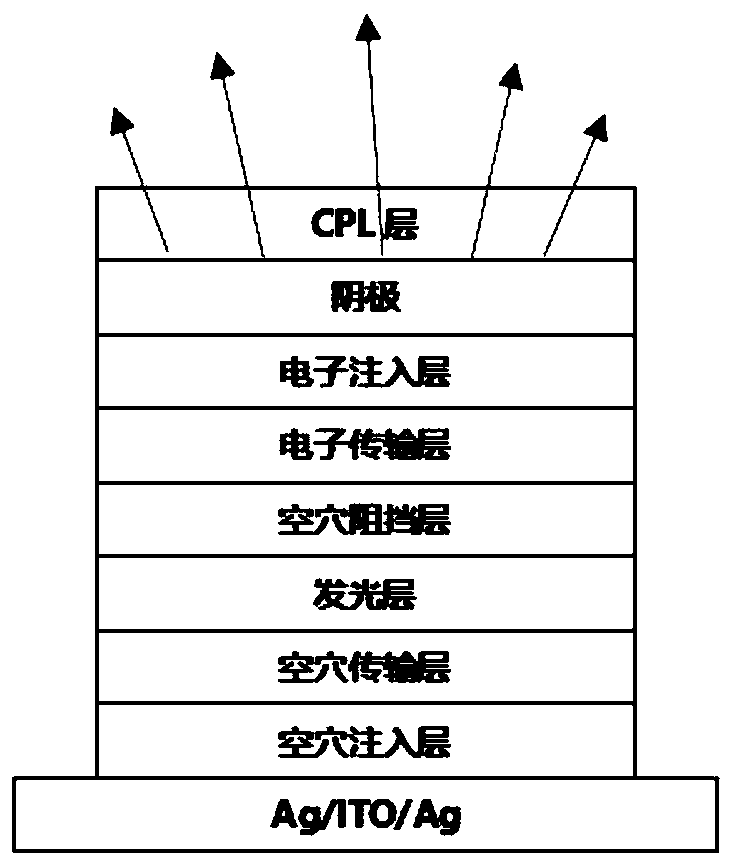
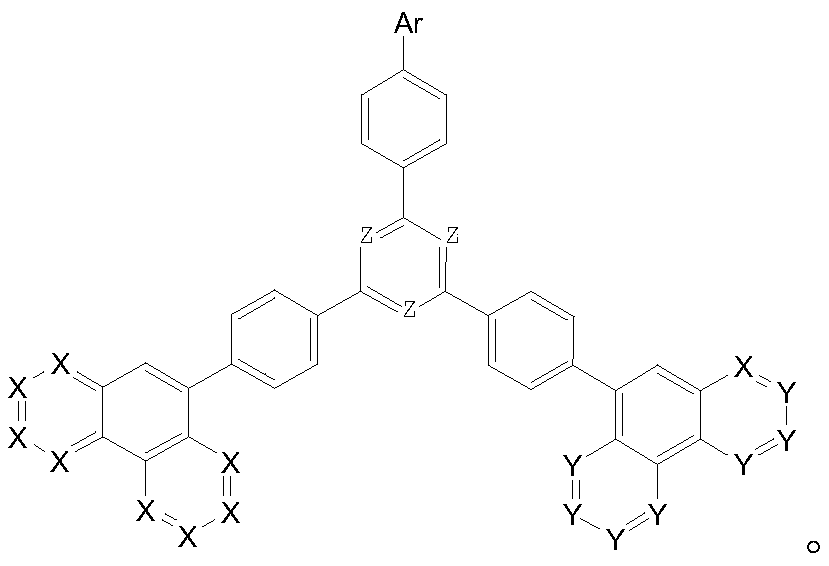
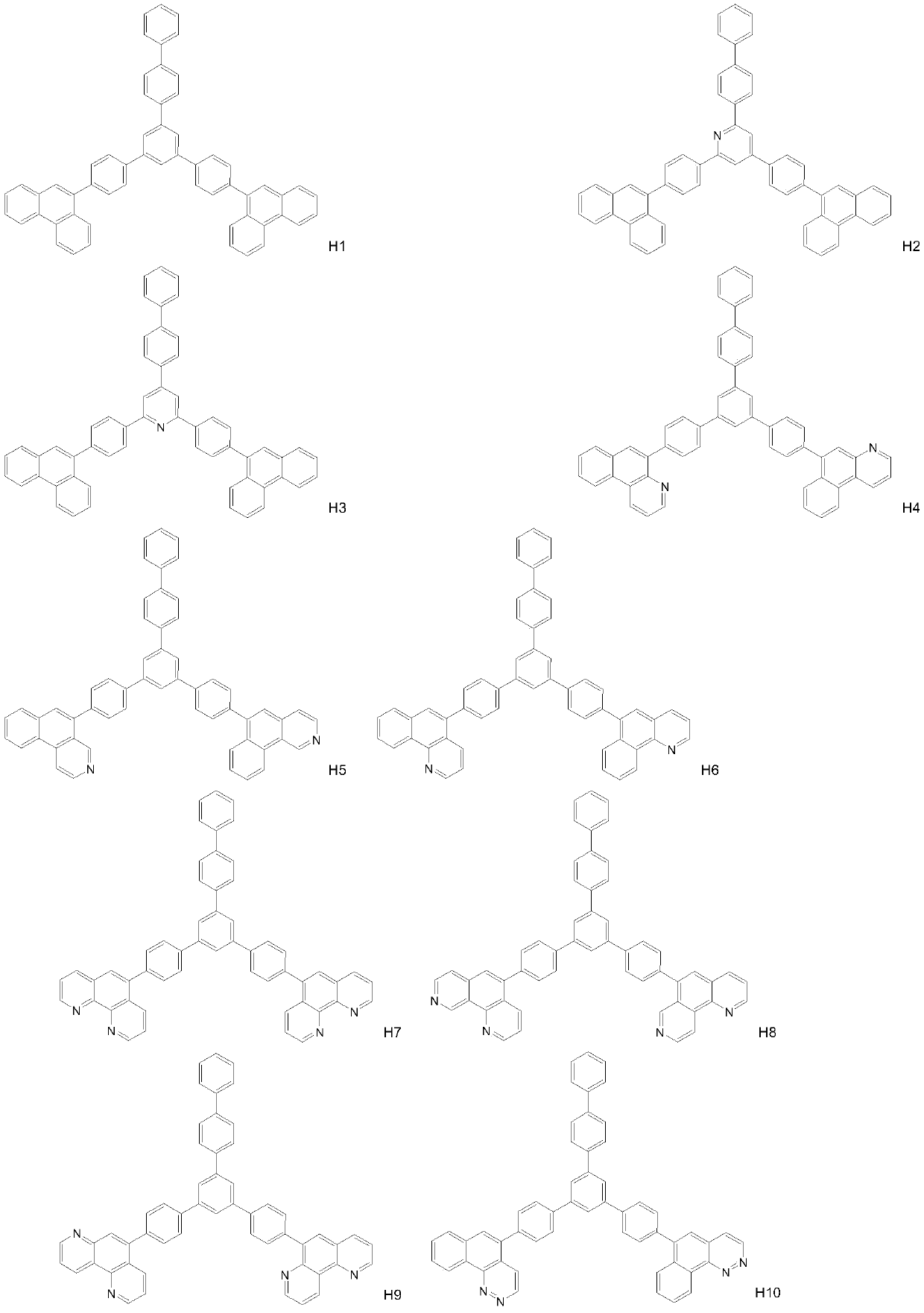
Compound taking phenanthroline and derivative thereof as core compound, and OLED device manufactured by taking compound as CPL layer
An o-phenanthroline and compound technology, applied in the field of OLED devices, can solve problems such as constraints, and achieve the effects of high glass transition temperature, low absorption and high refractive index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of compound H7
[0032]
[0033] 3,5 dibromo-1-(4-bromobiphenyl) terphenyl (0.1mol), 4-(9-phenanthroline) phenylboronic acid (0.05mol) joins in the 3L there-necked flask, adds potassium carbonate ( 0.075mol), toluene (1L), ethanol (500mL), deionized water (500mL), fully stirred, nitrogen protection, added tetrakistriphenylphosphine palladium (5mmol), slowly heated to reflux 110 ° C, kept for 24h, TLC detection The raw materials were completely reacted, cooled to room temperature, allowed to stand and separated, the organic phase was washed with saturated brine three times, dried over anhydrous sodium sulfate, concentrated to semi-dryness, added ethanol, a large amount of solids were produced, vacuum filtered, and the solids were dried in vacuum to obtain Compound H7. HPLC 99.2%, yield 78%. MS(EI) m / z: [M]+calcd for C54H34N4, 738.87; found, 738.28. Anal. Calcd for C54H34N4: C 87.78, H 4.64, N 7.58; found: C 87.75, H 4.86, N 7.39.
Embodiment 2
[0035] Synthesis of Compound H12
[0036]
[0037] Add 3,5-dibromo-1-(4-bromobiphenyl)terphenyl (0.1mol), 4-(9-(1,4diaza)phenanthrene)phenylboronic acid (0.05mol) into a 3L three-necked flask , add potassium carbonate (0.075mol), toluene (1L), ethanol (500mL), deionized water (500mL), stir well, nitrogen protection, add tetrakis triphenylphosphine palladium (5mmol), slowly heat up to reflux 110 ° C, Keep warm for 24 hours, TLC detects that the raw materials have reacted completely, cool down to room temperature, let stand to separate layers, wash the organic phase with saturated brine for 3 times, dry over anhydrous sodium sulfate, concentrate to semi-dryness, add ethanol, a large amount of solids are produced, and vacuum filter , the solid was dried in vacuo to obtain compound H12. HPLC 99.5%, yield 64%. MS(EI) m / z: [M]+calcd for C54H34N4, 738.87; found, 738.28. Anal. Calcd for C54H34N4: C 87.78, H 4.64, N 7.58; found: C 87.75, H 4.86, N7.39.
Embodiment 3
[0039] Synthesis of Compound H28
[0040]
[0041] 2,6 dibromo-4-(4-bromobiphenyl)pyridine (0.1mol), 4-(9-phenanthroline)phenylboronic acid (0.05mol) join in 3L there-necked flask, add potassium carbonate (0.075 mol), toluene (1L), ethanol (500mL), deionized water (500mL), fully stirred, under nitrogen protection, added tetrakistriphenylphosphine palladium (5mmol), slowly heated to reflux 110 ° C, kept for 24h, TLC detection of raw materials After the reaction is complete, cool down to room temperature, let stand to separate layers, wash the organic phase with saturated brine three times, dry over anhydrous sodium sulfate, concentrate to semi-dryness, add ethanol, a large amount of solid is produced, vacuum filter, and dry the solid in vacuum to obtain the compound H28. HPLC 99.5%, yield 90%. MS(EI) m / z: [M]+calcd for C53H33N5, 739.86; found, 739.27. Anal. Calcd for C53H33N5: C 86.04, H 4.50, N 9.47; found: C 86.26, H 4.36, N 9.39 。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com