Sustained release composition containing ticagrelor or pharmaceutically acceptable salt thereof
A slow-release composition and technology of ticagrelor, which can be used in drug combinations, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as the launch of slow-release products of ticagrelor, and achieve peak blood drug concentration Stable, reduce the risk of embolism, and reduce the effect of missed doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The composition of the present disclosure is prepared as follows:
[0052] First, ticagrelor (TGRL) or its pharmaceutically acceptable salt and copovidone (1:1) were dissolved in absolute ethanol, and spray-dried to prepare ticagrelor solid dispersion (TGRL-SD); then After the solid dispersion of ticagrelor, matrix forming agent, gel and other necessary excipients are mixed together, they are prepared into tablets through dry granulation and tabletting (die size is 19.2mm*10mm); some examples , the immediate release part is coated on the outer layer.
[0053] Release test method: take this product, according to the release test method (Chinese Pharmacopoeia 2015 edition four 0931 second method), the dissolution medium is 0.2% Tween 80 pH1.2 hydrochloric acid solution, after sampling, use UV-Vis spectrophotometry method for release testing.
[0054] In the dissolution test of some embodiments, at a specific time point, the tablet is taken out, the size of the tablet is...
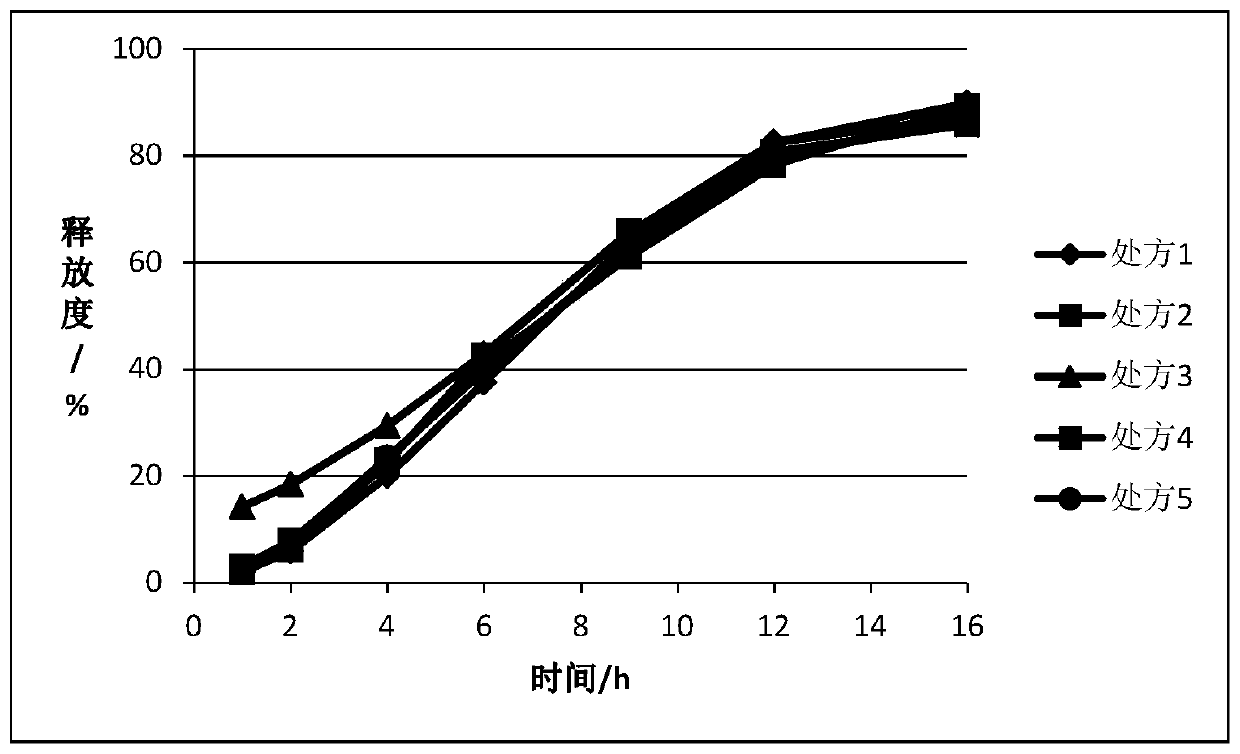
Embodiment 1
[0056] After mixing the ticagrelor solid dispersion, matrix forming agent, gel and other necessary excipients according to the prescription in Table 1, they were prepared into tablets by dry granulation and tabletting. In some embodiments, Coat the immediate release portion on the outer layer.
[0057] Table 1.
[0058]
[0059] Prescription 1 is composed of tablet cores of prescription 3. The cores of prescriptions 1 and 2 contain 93 mg of drug; the core of prescription 3 contains 93 mg of drug, and the outer coating layer contains 27 mg of drug; the core of prescriptions 4 and 5 contains 120 mg of drug.
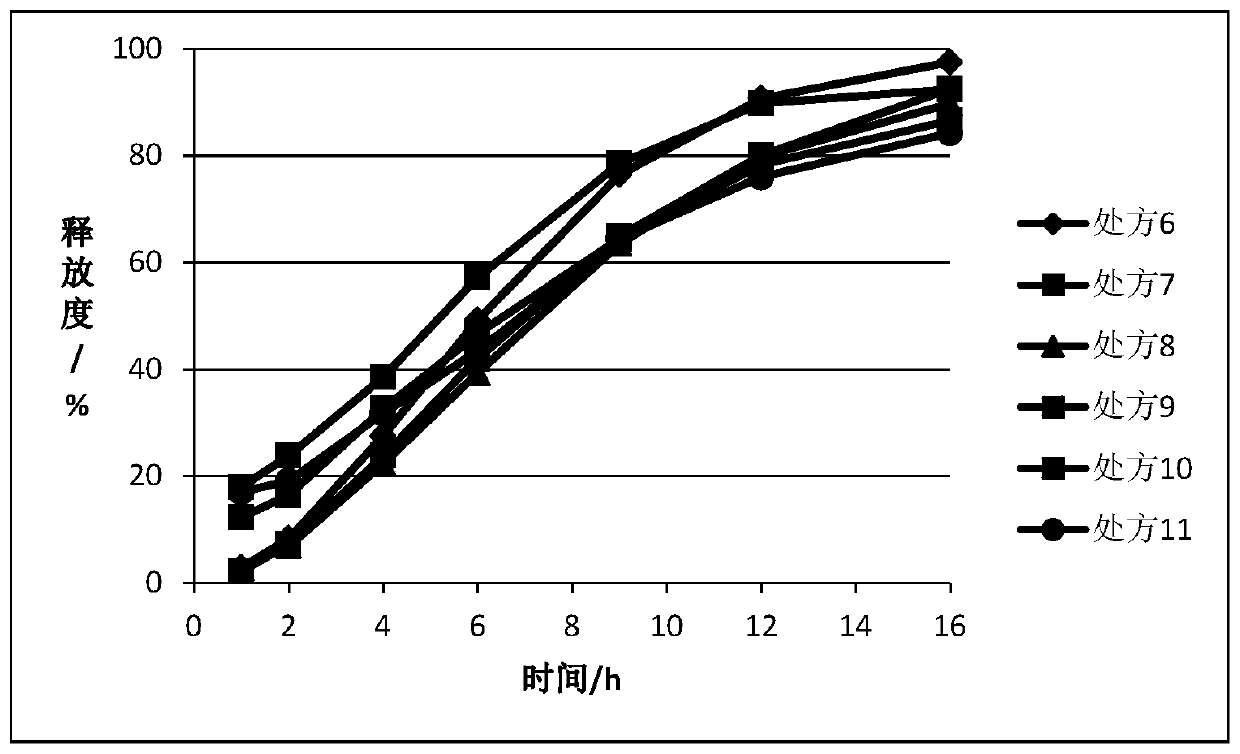
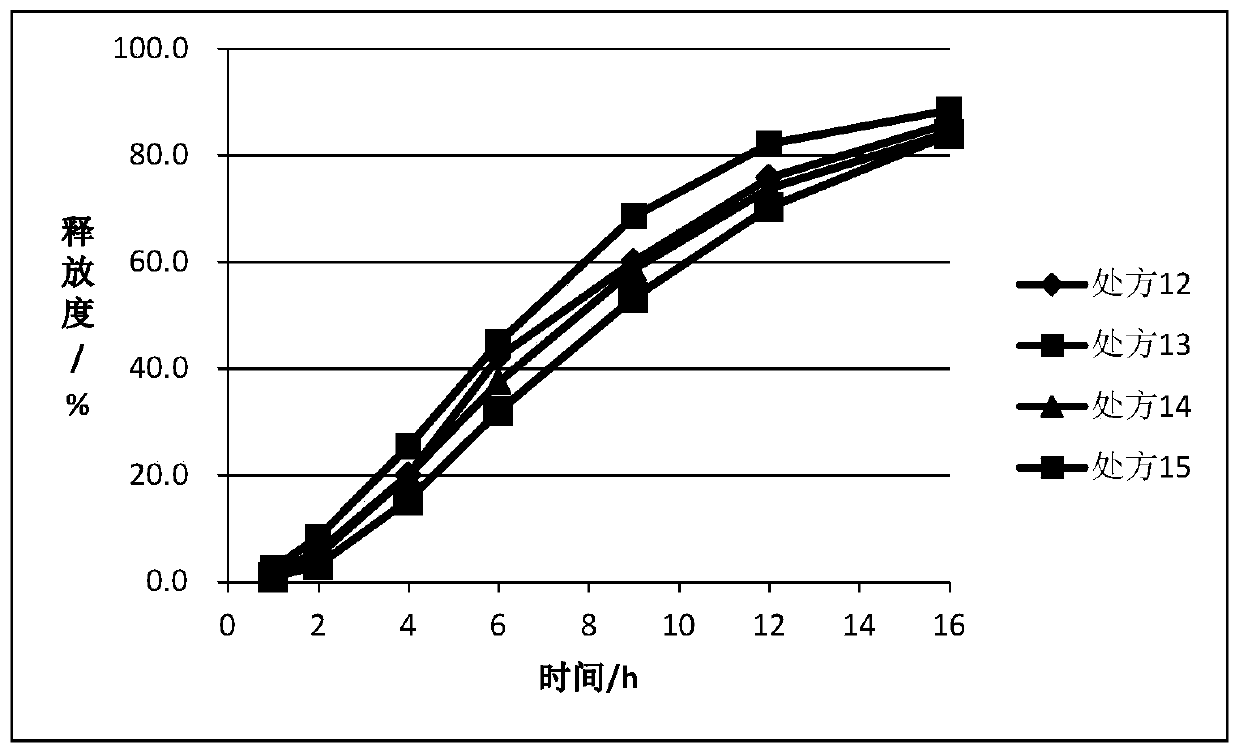
Embodiment 2
[0061] After the ticagrelor solid dispersion, matrix forming agent, gel and other necessary excipients are mixed according to the prescriptions in Table 2 and Table 3, they are prepared into tablets by dry granulation and tabletting. In an example, the immediate release portion is coated on the outer layer.
[0062] Table 2.
[0063]
[0064]
[0065] Prescriptions 6-8 are composed of the tablet cores of prescriptions 9-11 respectively. In prescription 9, the tablet core contains 140mg of drug, directly including the drug layer, and 40mg outside; in prescription 10, the tablet core is 140mg, with a PVA isolation layer, and then includes the drug layer, and the outer Contains 40mg; in prescription 11, the tablet core is 140mg, HPMC isolation layer, and then includes the drug layer, which contains 40mg.
[0066] table 3.
[0067]
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com