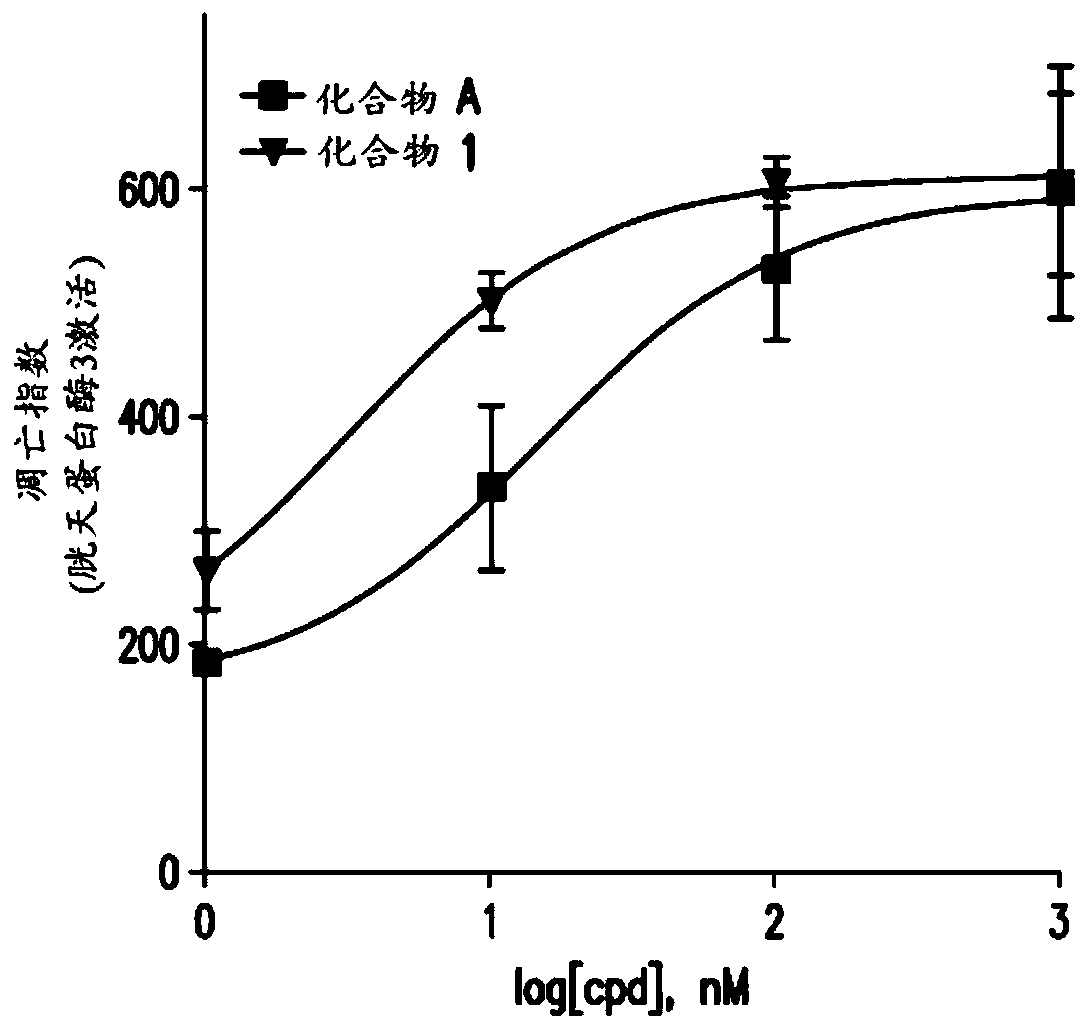
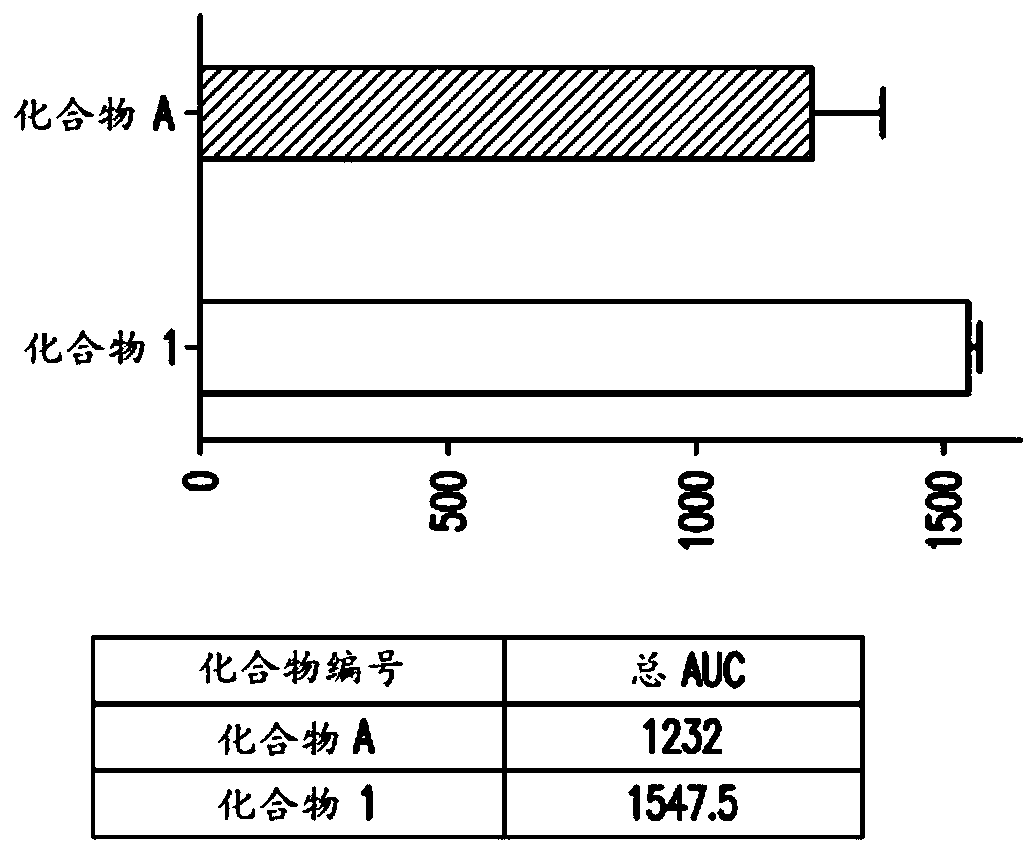
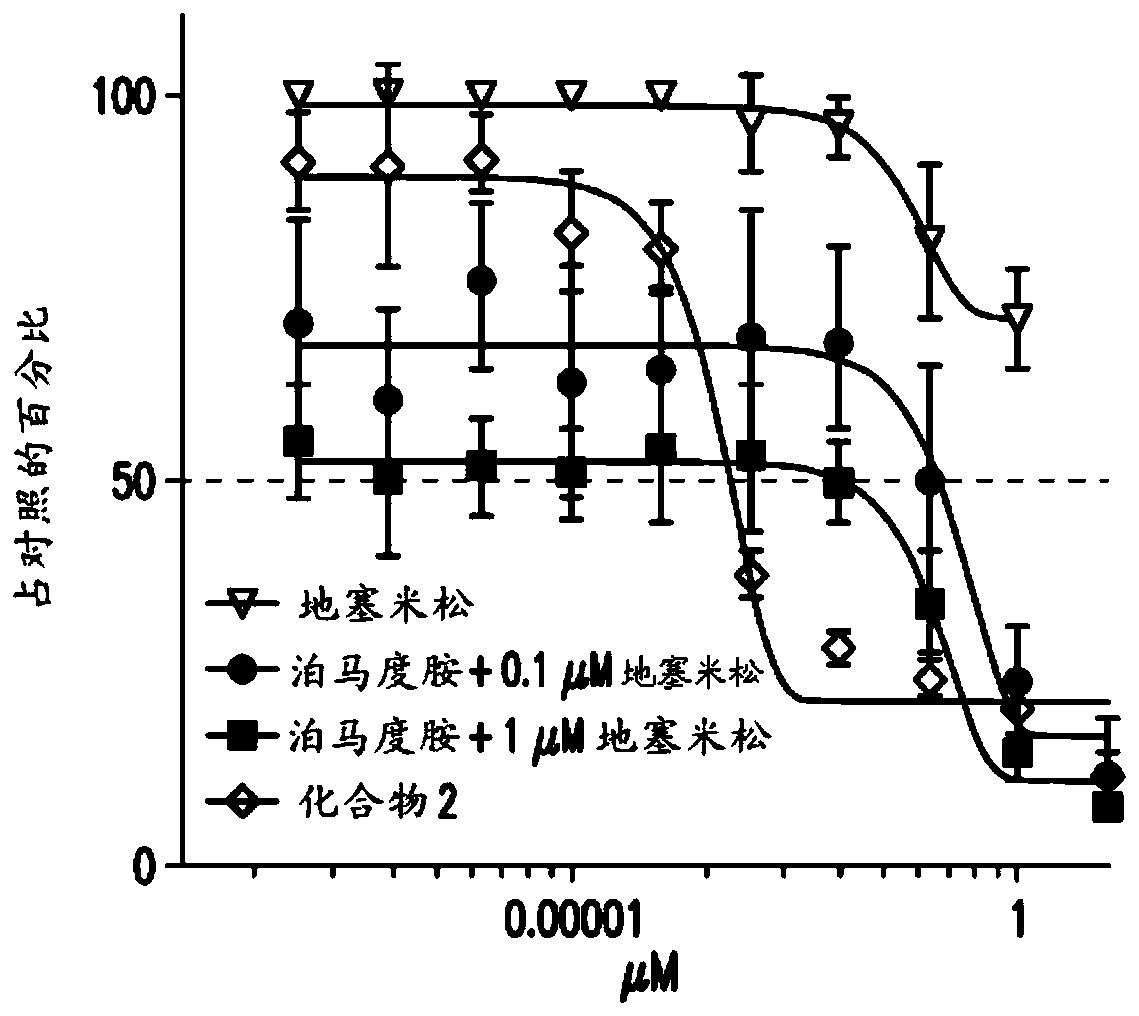
Antiproliferative compounds and methods of use thereof
A technology of compounds and compositions, applied in the fields of active ingredients of boron compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0396] Example 1: 4-(4-(4-(((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl)oxy)methanol yl)benzyl)piperazin-1-yl)-3-fluorobenzonitrile (Compound 1).
[0397]
[0398] 2-Amino-5-methoxy-5-oxopentanoic acid. To a suspension of 2-aminoglutaric acid (250 g, 1.70 mol) in dry methanol (2.5 L) was added trimethylsilyl chloride (277 g, 2.55 mol) under nitrogen over 30 minutes. The resulting clear solution was stirred at room temperature (20° C.) for 30 minutes. 1 H NMR indicated complete consumption of starting material. The reaction mixture was used in the next step without further work-up. 1 H NMR: 400MHz CD 3 ODδ: 4.17-4.15 (m, 1H), 3.71 (s, 3H), 2.70-2.60 (m, 2H), 2.33-2.25 (m, 2H).
[0399] 2-((tert-butoxycarbonyl)amino)-5-methoxy-5-oxopentanoic acid. To the above solution was added triethylamine (275 g, 2.72 mol) and di-tert-butyl dicarbonate (447.35 g, 2.05 mol). The mixture was stirred at 25°C for 2 hours. The solution was concentrated to dryness, then water (2....
Embodiment 2
[0410] Example 2: (S)-4-(4-(4-(((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl) Oxy)methyl)benzyl)piperazin-1-yl)-3-fluorobenzonitrile (Compound 2)
[0411]
[0412] (4S)-5-Amino-4-(benzyloxycarbonylamino)-5-oxo-pentanoic acid tert-butyl ester. To a solution of (2S)-2-(benzyloxycarbonylamino)-5-tert-butoxy-5-oxo-pentanoic acid (150g, 445mmol) in 1,4-dioxane (1.50L) Di-tert-butyl dicarbonate (155 g, 711 mmol), pyridine (70.3 g, 889 mmol) and ammonium bicarbonate (105 g, 1.33 mol) were added. The reaction mixture was stirred at 18°C for 16 hours, then concentrated. The residue was dissolved in ethyl acetate (5.0 L) and water (5.0 L), the organic layer was separated and washed with HCl (3.0 mL, 1 N), saturated sodium bicarbonate (3.0 L), brine (3.0 L), washed over Drying over anhydrous sodium sulfate, filtration and concentration afforded crude (4S)-5-amino-4-(benzyloxycarbonylamino)-5-oxo-pentanoic acid tert-butyl ester (450 g, crude) as a white solid, It was used i...
Embodiment 3
[0422] Example 3: (S)-4-(4-(4-(((2-(2,6-dioxopiperidin-3-yl)-1-oxoisoindoline-4-yl) Oxy)methyl)benzyl)piperazin-1-yl)-3-fluorobenzonitrile (compound 2)
[0423]
[0424]4-(4-(4-(Chloromethyl)benzyl)piperazin-1-yl)-3-fluorobenzonitrile hydrochloride. To a solution of 3-fluoro-4-(piperazin-1-yl)benzonitrile (100 g) in toluene (1400 mL) was charged acetic acid (28 mL) at 25 °C and the reaction mixture was maintained for 30 min. 4-(Chloromethyl)benzaldehyde (79 g) was charged at 25°C and the mixture was maintained for 2 hours. Sodium triacetoxyborohydride (52 g each) was charged 3 times every 30 minutes at 25°C. The mixture was stirred at 25°C for about 10 hours. Water (600 mL) was charged over 1 hour while maintaining the batch temperature below 30°C. Most of the lower layer was separated. The mixture was filtered, and the lower layer was separated. The organic layer was washed with water (200 mL). To the organic layer was charged IPA (75 mL), added 5-6N HCl in IPA (8 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com