P-N-containing porous organic cage ligand, preparation method and applications thereof
A technology of organic cages and ligands, applied in the fields of carbon monoxide reaction preparation, organic chemistry, organic compounds/hydrides/coordination complex catalysts, etc., to achieve unique catalytic performance, easy recovery, and good catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
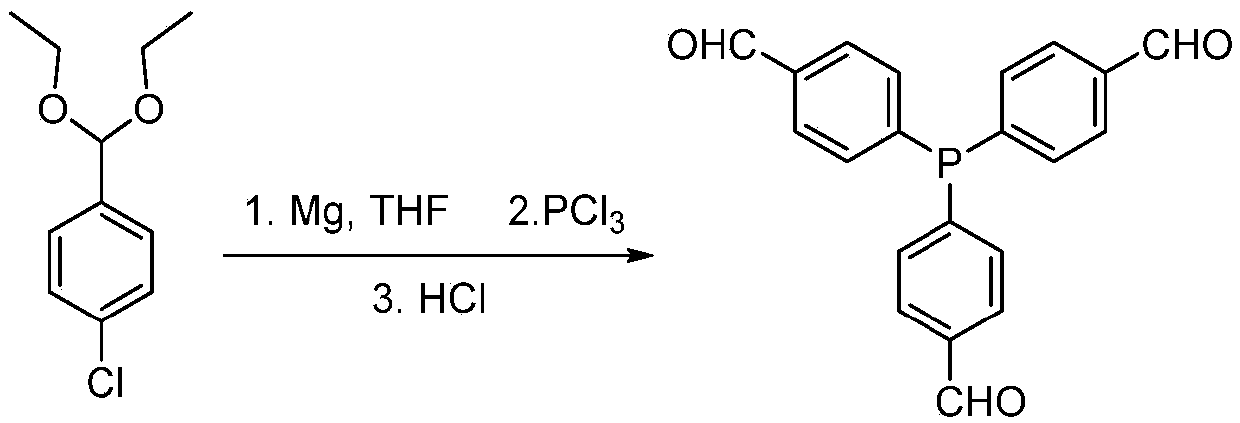
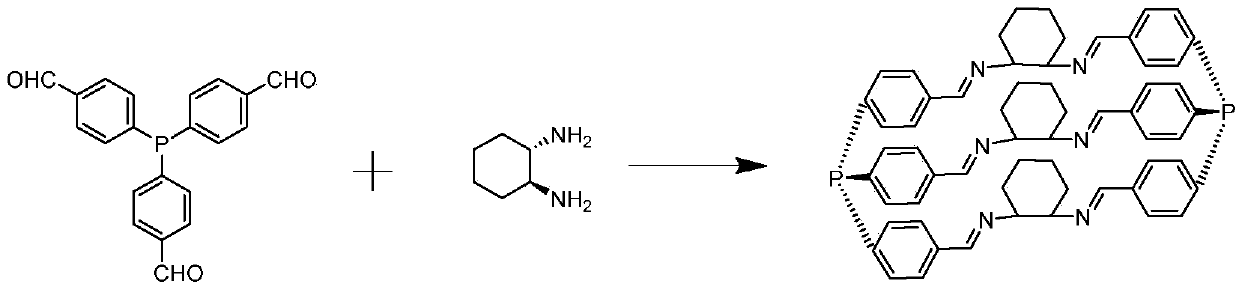
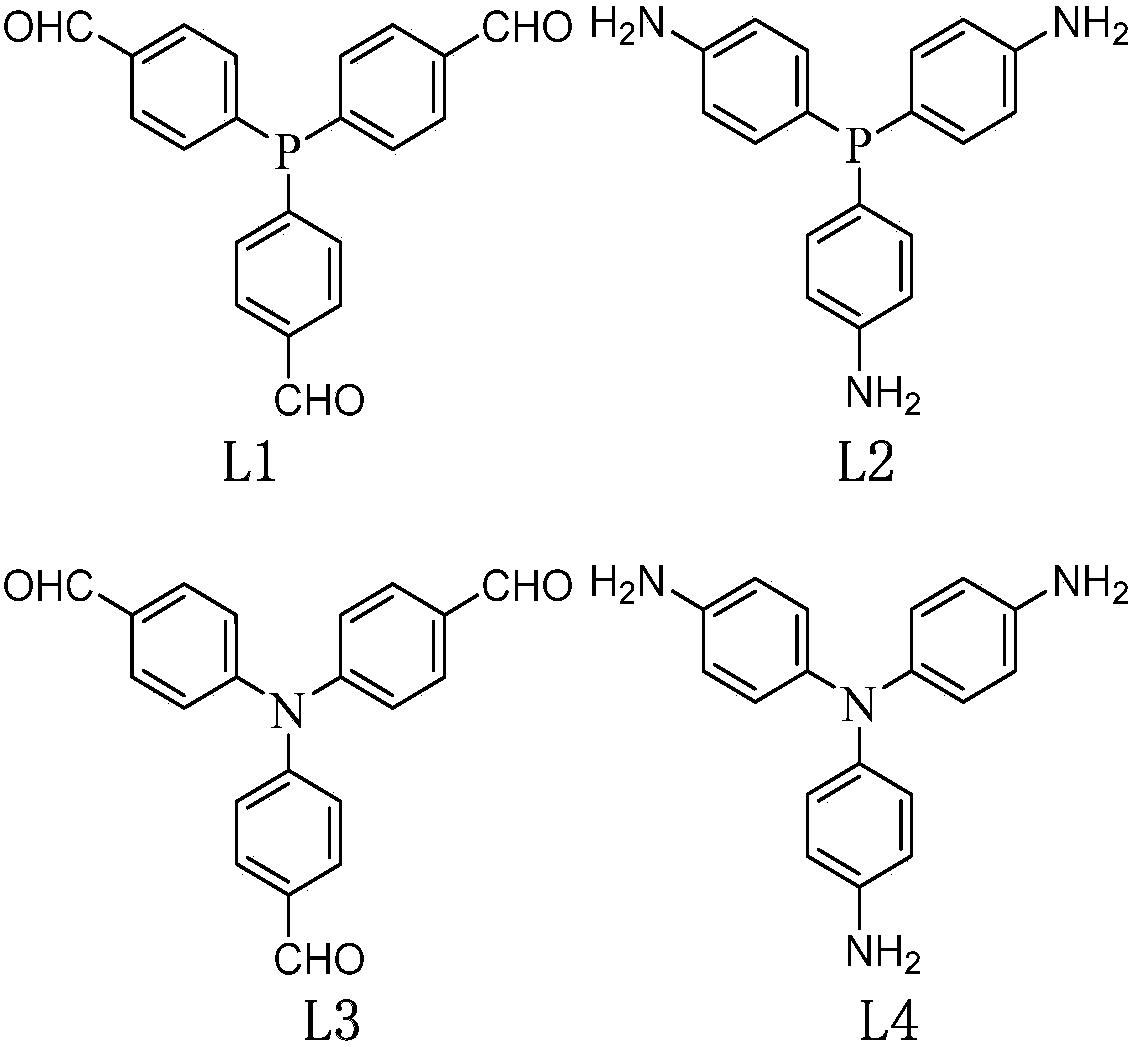
[0043] Aldehyde functionalized PPh 3 Ligand monomer (with image 3 Preparation of L1): Aldehyde functionalized PPh 3 The synthetic route of the ligand is attached figure 1 shown. 25g of 4-bromobenzaldehyde diacetic acid aldehyde (96mmol) was diluted 10 times (volume ratio) with tetrahydrofuran and slowly added dropwise to 4.4g of magnesium chips to prepare the Grignard reagent. 2.3 g of phosphorus trichloride was dissolved in 10 times (volume ratio) tetrahydrofuran solution and added dropwise to the prepared Grignard reagent. After fully reacting, an equal volume of 5% HCl solution was added to continue the reaction. After the reaction was completed, the oil phase was distilled off under reduced pressure to remove most of the solvent, and the eluent of 5:1 petroleum ether: ethyl acetate was passed through the column to obtain 6.5 g of a light yellow solid product with a yield of about 60%. attached Figure 4 , attached Figure 5 And attached Figure 6 The prepared aldeh...
Embodiment 2
[0047] In Example 2, in addition to weighing 2.12 grams of image 3 L57 is a co-monomer replacement with 2.12 grams attached image 3 Except L55 comonomer, all the other implementation processes are identical with embodiment 1.
Embodiment 3
[0049] In embodiment 3, except not adding acetic acid as catalyzer, all the other implementation processes are identical with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com