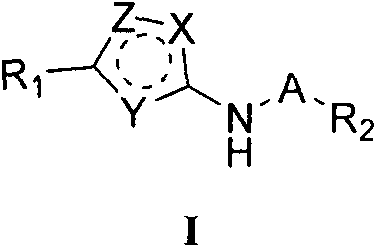
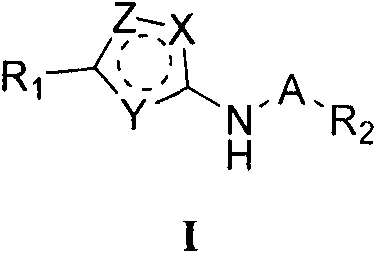
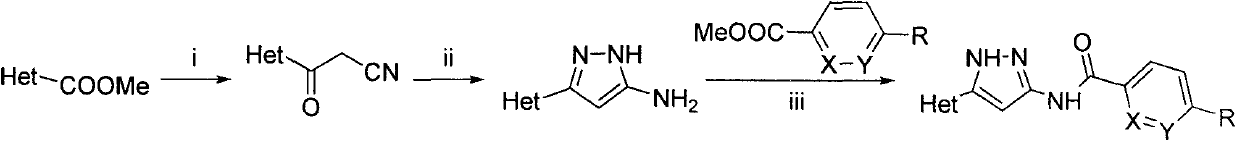
Oxygen-containing heterocyclic substituted azole compound and applications thereof
A compound, C1-C3 technology, applied in the field of medicinal chemistry, can solve problems such as weak selectivity of kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] N-(3-(Benzo[d][1,3]dioxol-5-yl)-1H-pyrazol-5-yl)-4-((1-methylpiperidine-4 -yl)amino)benzamide (1)
[0179] Into a round-bottomed flask were added sequentially tetrahydrofuran (12ml), acetonitrile (5mL), sodium hydride (0.17g, 7.1mmol) and benzo[d][1,3]dioxole-5 under ice-bath conditions. -Methyl formate (0.43g, 2.4mmol), stirred for 0.5h and then heated to reflux, reacted for 5h. The reaction solution was poured into ice-water mixture (30ml), and the pH was adjusted to 2. A large amount of yellow solid precipitated, which was filtered and dried to obtain a crude product. After the crude product was subjected to column chromatography (PE:EA=2:1), 0.413 g of a light yellow solid was obtained, with a yield of 91%. MS[M-H] + 188.03.
[0180] The product obtained in the previous step, hydrazine hydrochloride (0.45g, 4.4mmol), triethylamine (0.44g, 4.4mmol) and ethanol (10ml) were sequentially added into a 25mL round bottom flask, and refluxed for 8h. The reaction soluti...
Embodiment 2
[0183] N-(3-(Benzo[d][1,3]dioxol-5-yl)-1H-pyrazol-5-yl)-4-(4-methylpiperazine-1- base) benzamide (2)
[0184] The preparation method is similar to (1), and a light yellow solid is obtained. 1 H NMR (300MHz, CDCl3) δ12.7 (s, 1H), 10.3 (s, 1H), 7.64-7.57 (m, 2H), 7.35 (d, 1H), 7.22 (d, 1H), 7.06-6.99 ( m, 2H), 6.88-6.81(m, 2H), 6.07(s, 2H), 3.20(t, 4H), 2.98(t, 4H), 2.60(s, 3H). MS(m / z): [ M+H] + 406.2.
Embodiment 3
[0186] N-(3-(Benzo[d][1,3]dioxol-5-yl)-1H-pyrazol-5-yl)-4-(piperidin-1-yl)benzyl Amides (3)
[0187] The preparation method is similar to (1), and a light yellow solid is obtained. 1 H NMR (300MHz, CDCl3) δ12.7(s, 1H), 10.3(s, 1H), 7.59-7.52 (m, 2H), 7.25(d, 1H), 7.19(d, 1H), 7.01(d, 1H), 6.89-6.82(m, 2H), 6.52(s, 1H), 6.07(s, 2H), 3.49-3.43(m, 4H), 1.67-1.57(m, 6H).MS(m / z) : [M+H] + 391.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com