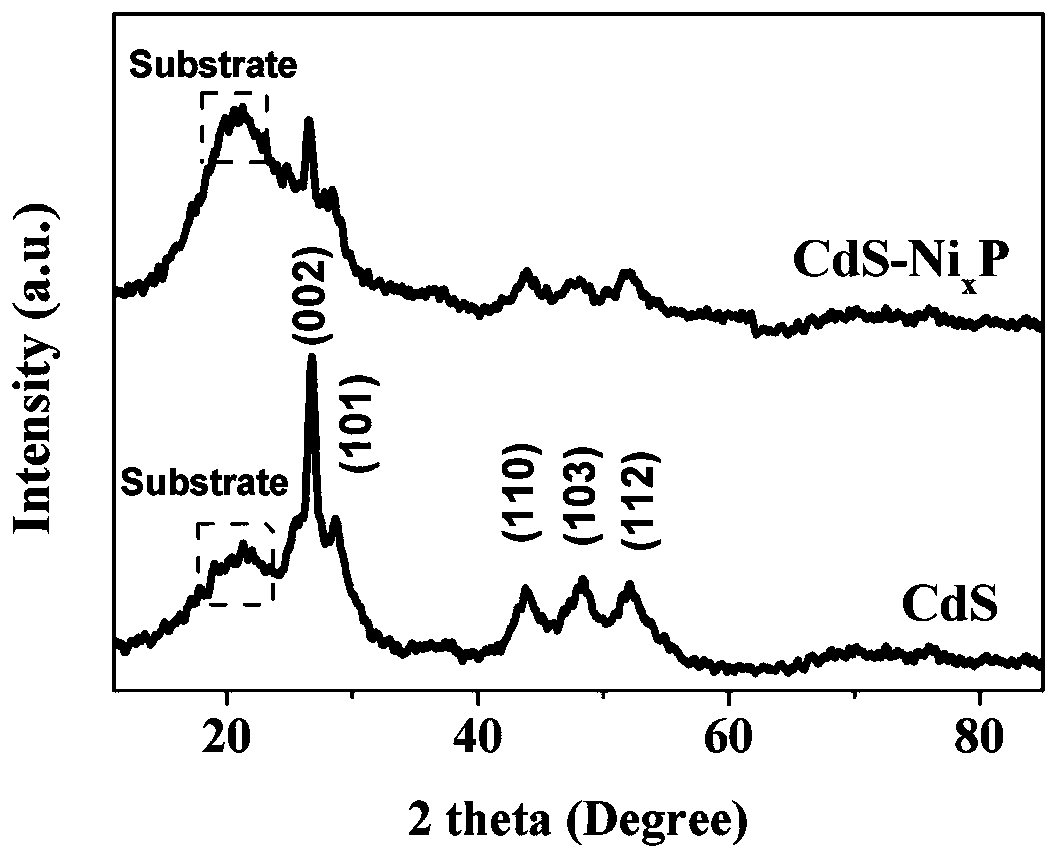
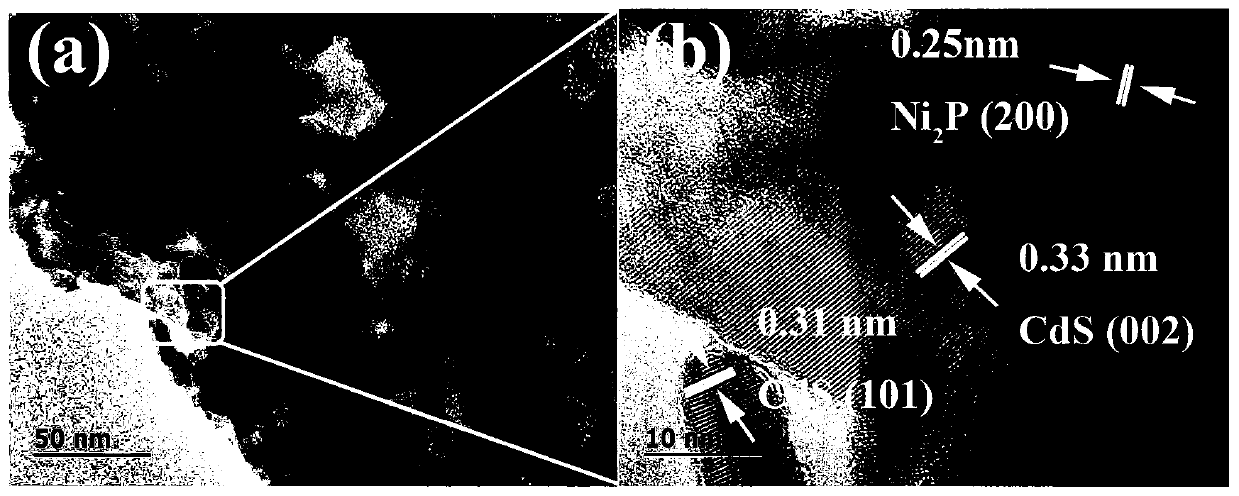
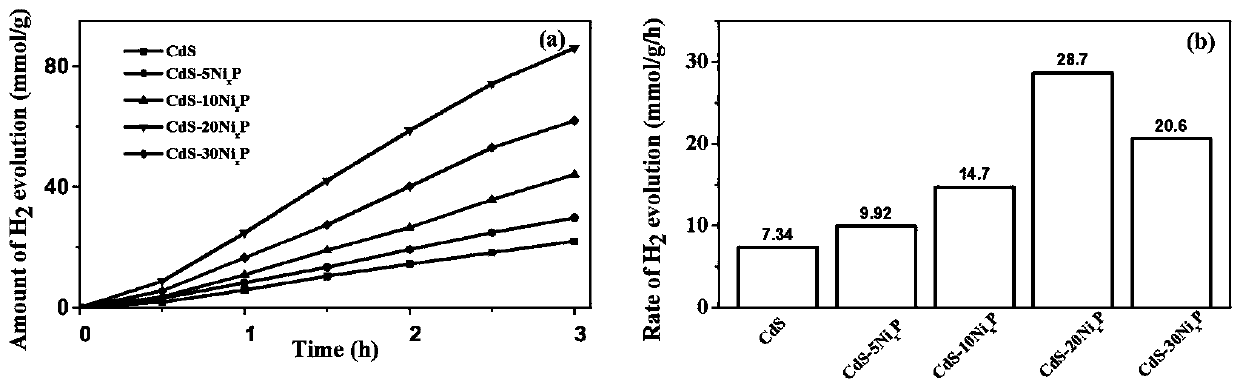
Preparation method of nickel phosphide cocatalyst by photodeposition on cadmium sulfide
A co-catalyst and cadmium sulfide technology, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as hindering large-scale application, harsh experimental conditions, and complicated preparation methods, and achieve the goal of improving visible light. Catalytic activity, simple preparation method, and the effect of inhibiting recombination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] A preparation method for photodepositing nickel phosphide cocatalyst on cadmium sulfide, comprising the following steps:
[0019] (1) Preparation of cadmium sulfide nanosheets: Dissolve 0.183g cadmium chloride and 0.16g sulfur powder in 30ml diethylenetriamine, stir evenly for 30 minutes and transfer it to the high pressure of 50ml polytetrafluoroethylene liner kettle, and heated at 80°C for 48 hours, after natural cooling, fully cleaned with deionized water and ethanol, and dried at 60°C to obtain cadmium sulfide nanosheets;
[0020] (2) Preparation of cadmium sulfide nanosheets / nickel phosphide: the cadmium sulfide nanosheets in step (1) are configured into a cadmium sulfide solution, and the cadmium sulfide solution is added to the aqueous solution containing nickel chloride and sodium hypophosphite, and The mixed solution is transferred into a reactor, and after the oxygen in the solution is extracted, it is irradiated with a simulated sunlight light source to obtai...
Embodiment 1
[0024] Dissolve 0.183g of cadmium chloride and 0.16g of sulfur powder in 30ml of diethylenetriamine, stir evenly for 30 minutes, transfer it to an autoclave with a 50ml polytetrafluoroethylene liner, and heat at 80°C for 48 hours, After natural cooling, fully wash with deionized water and ethanol, and dry at 60°C to obtain cadmium sulfide nanosheets, which are designated as cadmium sulfide-1 photocatalyst.
Embodiment 2
[0026] Dissolve 0.183g of cadmium chloride and 0.16g of sulfur powder in 30ml of diethylenetriamine, stir evenly for 30 minutes, transfer it to an autoclave with a 50ml polytetrafluoroethylene liner, and heat at 80°C for 48 hours, After natural cooling, fully wash with deionized water and ethanol, and dry at 60°C to obtain cadmium sulfide nanosheets.
[0027] Ultrasonic disperse 0.02g of cadmium sulfide nanosheets into 20ml of deionized water, disperse 0.019g of nickel chloride and 0.059g of sodium hypophosphite into 15ml of deionized water respectively, and mix them with cadmium sulfide aqueous solution to turn them into photocatalytically active In the reactor of the fully automatic online evaluation system, use a mechanical pump to empty the air in the reactor and use a 300-watt xenon lamp as the light source to photodeposit nickel phosphide for 5 minutes to obtain cadmium sulfide / nickel phosphide-2 photocatalyst .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com