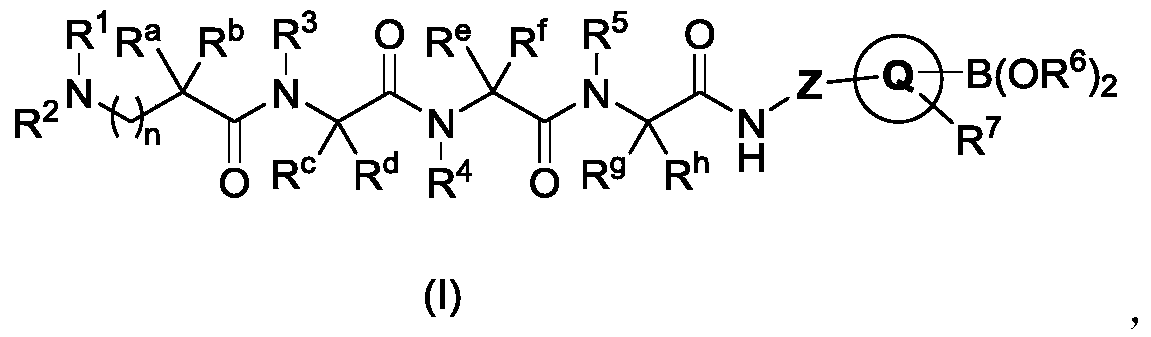
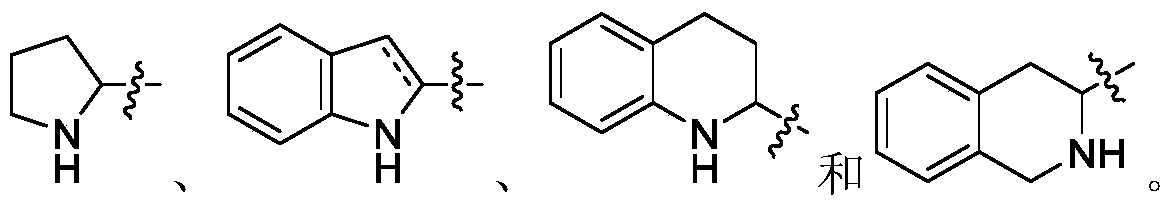
Polypeptide compound, preparation, medicine composition, preparation method and application
A technology of compounds and polypeptides, applied in the field of medicine, can solve the problem of not easy tolerance, and achieve the effects of less side effects, good analgesic activity, and low brain permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of Polypeptide Compound TM-1
[0066] Step 31: Int-1 (0.545 g, 0.622 mmol), HOBT (0.201 g, 1.492 mmol), HBTU (0.566 g, 1.492 mmol), and DIEA (0.320 g, 2.488 mmol) were dissolved in DCM (15 mL) at room temperature Under stirring for 0.5h, then add the crude product of Int-4 and react at room temperature for 2h. The reaction solution was washed with saturated ammonium chloride solution, water and saturated brine respectively, dried over anhydrous sodium sulfate, and the crude product after filtration and concentration was purified by Prep-HPLC to obtain intermediate compound 1-1;
[0067] Step 32: Add TFA (1 mL) dropwise to a solution of 1-1 (500 mg) in DCM (2 mL), stir at room temperature for 1 h, and purify the crude product after concentration to dryness by Prep-HPLC to obtain the trifluoropolypeptide compound TM-1 acetate.
[0068] The mass spectrometry and NMR characterization of the prepared TM-1 are as follows:
[0069] ESI-MS(m / z):665.4(M+H + )
...
Embodiment 2
[0081] Preparation of polypeptide compound TM-2
[0082] This embodiment is based on embodiment 1, and the difference with embodiment 1 is:
[0083] The dosage of Int-1 is 0.093g; Int-4 is replaced by pyrrole-3-boronic acid pinacol ester; the synthesis of pyrrole-3-boronic acid pinacol ester is similar to Int-4, by N-tert-butoxycarbonyl-pyrrole -3-Boronic acid pinacol ester is obtained by removing the protecting group under acidic conditions. The obtained crude product was purified by Prep-HPLC to obtain 13.3 mg of the trifluoroacetate salt of the target compound TM-2.
[0084] The mass spectrometry and NMR characterization of the prepared TM-2 are as follows:
[0085] ESI-MS(m / z):651.4(M+H + )
[0086] 1H NMR (400MHz, DMSO): δ8.76-8.73(m,1H),8.38–8.16(m,2H),8.02(s,3H),7.70(s,3H),7.39–7.13(m,10H) ,4.69-4.64(m,1H),4.52-4.43(m,2H),4.02(s,1H),3.44–3.25(m,3H), 3.14-3.02(m,3H),2.96-2.90(m, 1H),2.87–2.66(m,3H),2.01-1.92(m,1H),1.71–1.29(m,11H), 0.92-0.87(m,6H).
Embodiment 3
[0088] Preparation of peptide compound TM-3:
[0089] This embodiment is based on embodiment 1, and the difference with embodiment 1 is:
[0090] The dosage of Int-1 is 0.090g, replace Int-4 with 2,5-dihydro-1H-pyrrole-3-boronic acid pinacol ester; 2,5-dihydro-1H-pyrrole-3-boronic acid pinacol ester The synthesis of the ester is similar to that of Int-4, which can be obtained by deprotecting N-tert-butoxycarbonyl-2,5-dihydro-1H-pyrrole-3-boronic acid pinacol ester under acidic conditions. The obtained crude product was purified by Prep-HPLC to obtain 13.3 mg of the trifluoroacetate salt of the target compound TM-3.
[0091] The mass spectrometry and NMR characterization of the prepared TM-3 are as follows:
[0092] ESI-MS(m / z):649.4(M+H + )
[0093] 1H NMR (400MHz, CD 3 OD):δ7.44–7.12(m,10H),6.58–6.34(m,1H),4.77–4.52(m,3H), 4.48-4.25(m,4H),4.11-4.06(m,1H), 3.28–3.18(m,1H),3.06–2.89(m,4H),1.88-1.80(m,1H), 1.79–1.38(m,8H),1.02–0.91(m,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com