Preparation method and applications of a class of heterocyclic compounds with immunomodulatory effect
A compound and hydrate technology, applied in the field of small molecule protein inhibitors, can solve the problems of high production cost, easy immunogenicity, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
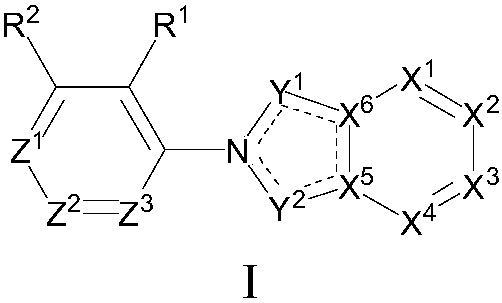
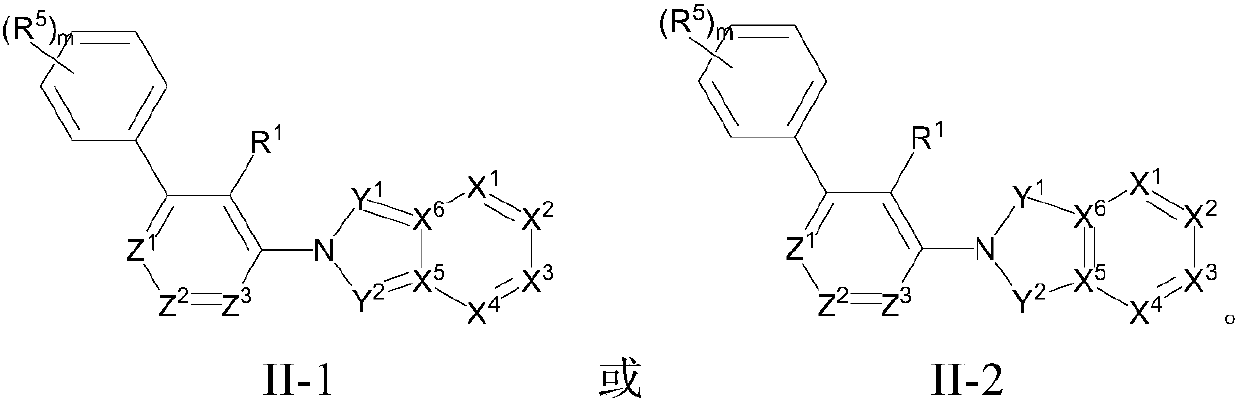
[0096] The preparation of formula I compound
[0097] The present invention also provides a method for preparing the compound as described in the first aspect of the present invention, the method comprising step (1) or (2):
[0098]
[0099] Compound 1 is removed from Boc protection under acidic conditions to obtain compound 2, and then compound 3 is reacted with compound 3 under the action of a palladium catalyst in the presence of a base and a phosphine compound to obtain compound 4, compound 4 is reduced to obtain compound 5, and then reductive amination Reaction obtains the compound shown in formula I.
[0100] Pharmaceutical compositions and methods of administration
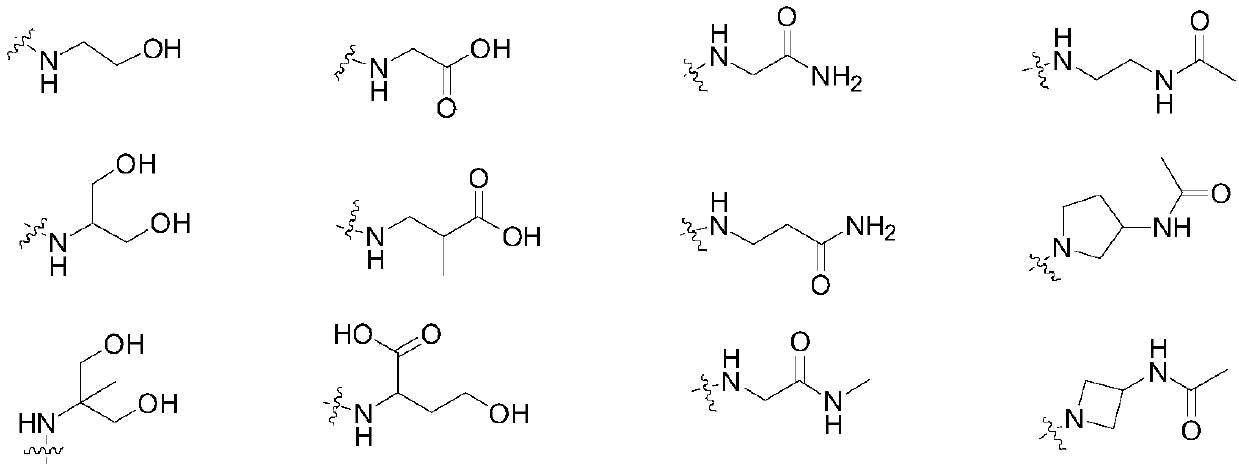
[0101] Since the compound of the present invention has excellent inhibitory activity against PD-1, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, and compounds containing the present invention are the main ...
Embodiment 1
[0165] 2-(((2-(2-Chloro-[1,1'-biphenyl]-3-yl)-2H-pyrazolo[3,4-b]pyridin-5-yl)methyl)amino ) Ethan-1-ol
[0166]
[0167] 2-(2-Chloro-[1,1’-biphenyl]-3-yl)-2H-pyrazolo[3,4-b]pyridine-5-carbaldehyde.
[0168]
[0169] To 2-(3-bromo-2-chlorophenyl)-2H-pyrazolo[3,4-b]pyridine-5-carbaldehyde (50mg, 0.15mmol), 1 , to a solution of 4-dioxane (3 mL) and water (0.5 mL) was added [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (11 mg, 0.015 mmol) and carbonic acid Potassium (62mg, 0.45mmol), under the protection of nitrogen, the reaction solution was heated to 100 degrees and stirred for one hour; TLC showed that the raw material disappeared completely, the reaction solution was filtered, the filter cake was washed twice with methanol, the filtrate was collected, concentrated, and the residue The title compound 2-(2-chloro-[1,1'-biphenyl]-3-yl)-2H-pyrazolo was obtained by normal phase flash separation and purification (petroleum ether:ethyl acetate=50:1) [3,4-b]pyrid...
Embodiment 2
[0177]1-((2-(2-Chloro-[1,1'-biphenyl]-3-yl)-2H-pyrazolo[3,4-b]pyridin-5-yl)methyl)piperidine -2-carboxylic acid
[0178]
[0179] 1-((2-(2-Chloro-[1,1'-biphenyl]-3-yl)-2H-pyrazolo[3,4-b]pyridin-5-yl)methyl)piperidine -2-carboxylic acid
[0180] To Example 1A (25mg, 0.075mmol), piperidine-2-carboxylic acid (19mg, 0.15mmol) in N,N-dimethylformamide (3mL) solution was added catalytic amount of acetic acid (1 drop), the reaction The solution was heated to 60 degrees and stirred for half an hour, then sodium cyanoborohydride (14mg, 0.23mmol) was added, and the stirring was continued overnight; filtered, concentrated, and the residue was purified by preparative high performance liquid chromatography to obtain the target product 1-((2- (2-Chloro-[1,1'-biphenyl]-3-yl)-2H-pyrazolo[3,4-b]pyridin-5-yl)methyl)piperidine-2-carboxylic acid ( 8 mg, 73%) as a white solid.
[0181] MS(ESI):m / z=447.1[M+H] + .
[0182] 1 H NMR (400MHz, DMSO-d 6 )δ8.79 (s, 1H), 8.67 (d, J = 2.2Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com