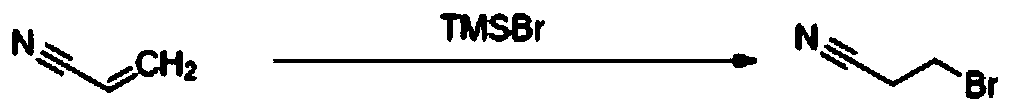Preparation method of 3-bromopropionitrile
A technology of bromopropionitrile and acetonitrile, applied in the field of preparation of 3-bromopropionitrile, can solve the problems of high raw material cost, unfriendly environment, low yield and the like, and achieves high material utilization rate, simple post-processing and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A kind of preparation method of 3-bromopropionitrile of the present invention, concrete steps are as follows: dissolve trimethylbromosilane (71.40kg, 653.60mol, 0.95eq) in acetonitrile (15Vol), start stirring; Under nitrogen protection, 20~40 Add acrylonitrile (25.40kg, 479.25mol, 1eq) dropwise at ℃; add deionized water (10.60kg, 294.44mol, 2eq) dropwise at a controlled reaction temperature of 20-40°C after dropping, and react overnight at 20-40°C Until the raw material disappears substantially, the precipitated solid is filtered, the filtrate is concentrated under reduced pressure until almost no solvent flows out, and the rectification device (75 ± 5°C, 9-12mmTor) is used for rectification to obtain 45.45kg of colorless liquid with a yield of 70.9% ( Gas chromatographic purity greater than 98%). H NMR (CDCl 3 ,400MHz): δ3.52-3.55(t,3H),2.98-3.01(t,2H). Synthetic route of the present invention such as figure 1 shown.
Embodiment 2
[0029] A preparation method of 3-bromopropionitrile of the present invention, the specific steps are as follows: dissolve trimethylbromosilane (71.40g, 0.66mol, 0.95eq) in toluene (12Vol), start stirring; under nitrogen protection, 20-40 Add acrylonitrile (25.40g, 0.48mol, 1eq) dropwise at ℃; add deionized water (10.60g, 0.29mol, 2eq) dropwise at a controlled reaction temperature of 20-40°C after the dropwise addition, and react overnight at 20-40°C Until the raw material disappears substantially, the precipitated solid is filtered, the filtrate is concentrated under reduced pressure until there is no solvent to flow out, and the rectification device (75 ± 5°C, 9-12mmTor) is used for rectification to obtain 38.0g, yield 59.7% (gas chromatographic purity greater than 98%). H NMR (CDCl 3 ,400MHz): δ3.52-3.55(t,3H),2.98-3.01(t,2H).
[0030] Comparing Example 1 and Example 2, it can be seen that the yield of using acetonitrile as the organic solvent is greater than that of tolue...
Embodiment 3
[0032] A preparation method of 3-bromopropionitrile of the present invention, the specific steps are as follows: dissolve trimethylbromosilane (71.40g, 0.66mol, 0.95eq) in acetonitrile (15Vol), and start stirring. Under the protection of nitrogen, add acrylonitrile (25.40g, 0.48mol, 1eq) dropwise at 0-20°C; after the addition, control the reaction temperature at 0-20°C and add deionized water (10.60g, 0.29mol, 2eq) dropwise, After the dropwise addition is completed, react at 10-20°C overnight until the raw materials basically disappear, filter the precipitated solid, concentrate the filtrate under reduced pressure until almost no solvent flows out, and use a rectification device (75±5°C, 9-12mmTor) to rectify to obtain 41.0 g, yield 64.2% (gas chromatography purity greater than 98%). H NMR (CDCl 3 ,400MHz): δ3.52-3.55(t,3H),2.98-3.01(t,2H).
[0033] Comparing Example 1 and Example 3, it can be seen that the yield will change at different reaction temperatures. In the present...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com