A kind of method for extracting calcium without saponification
A non-saponifiable and extraction technology, applied in the field of non-saponifiable calcium extraction, can solve the problems of low extraction rate, increase in impurities, increase in cost, etc., and achieve the effects of improving product purity, high extraction rate and reducing loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A method for extracting calcium without saponification, comprising the steps:
[0029] (1) adjusting the pH of the nickel-cobalt-manganese leaching solution to be 3.1;
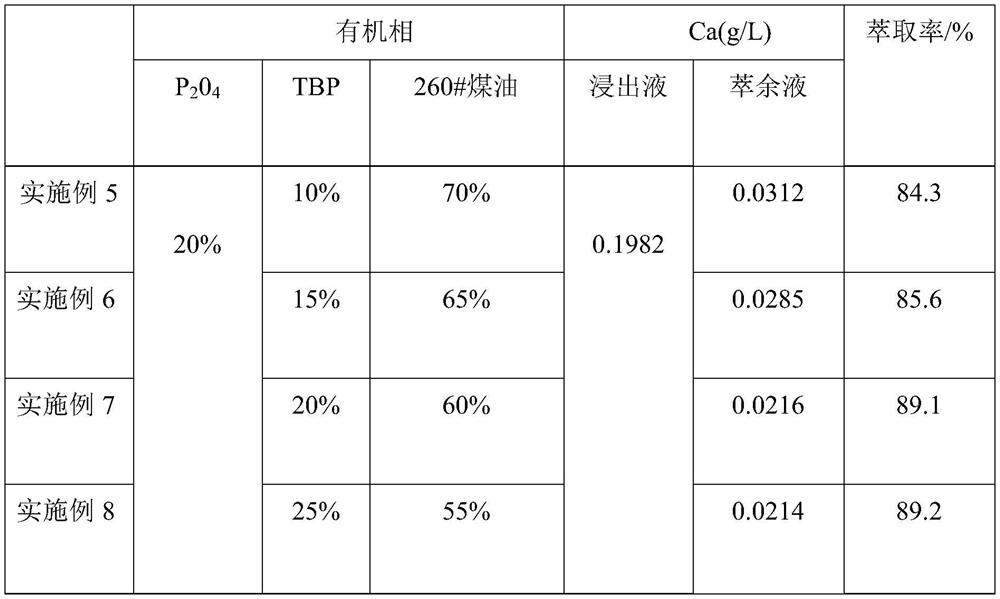
[0030] (2) 20% P204 (bis(2-ethylhexyl) phosphate), 20% TBP (tributyl phosphate) and 60% sulfonated kerosene by mass percentage are made into an organic phase, and 100 mL of the organic phase and 100 mL of The nickel-cobalt-manganese leaching solution was placed in a separatory funnel, oscillated at a rotational speed of 300 rpm, extracted at a temperature of 25 °C for 8 min, left to stand for stratification for 45 s, and then separated from the water phase to obtain an organic phase containing calcium ions.
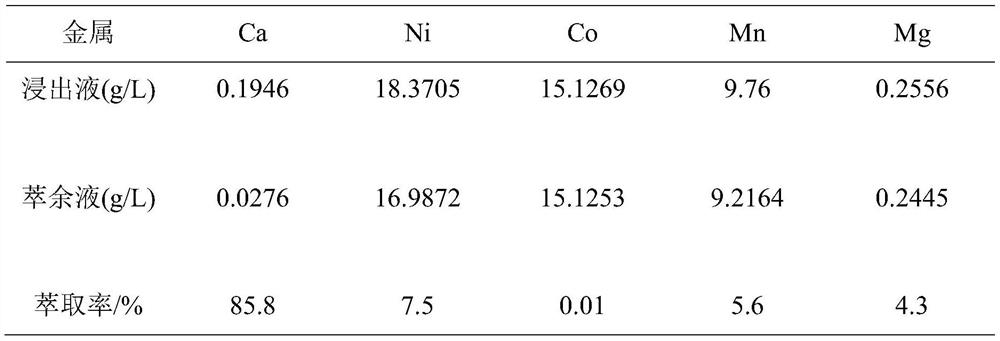
[0031] The detection results of the content of calcium and other metals in the raffinate are shown in Table 1. The extraction rate of calcium is 85.8%, the extraction rates of the main metals nickel, cobalt, and manganese are 7.5%, 0.01%, and 5.6%, respectively, and the extraction rate of magnesium...
Embodiment 2
[0035] A method for extracting calcium without saponification, comprising the steps:
[0036] (1) adjusting the pH of the nickel-cobalt-manganese leaching solution to be 5.5;
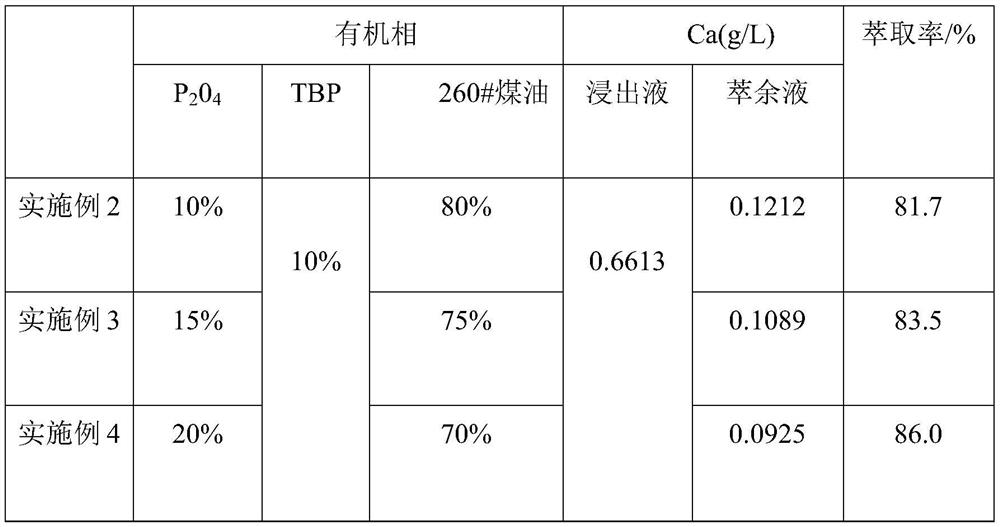
[0037] (2) 10% P204 (bis(2-ethylhexyl) phosphate), 10% TBP (tributyl phosphate) and 80% sulfonated kerosene are prepared into organic phase by mass percentage, take 100 mL of organic phase and 100 mL of The nickel-cobalt-manganese leaching solution was placed in a separatory funnel, oscillated at a rotational speed of 300 rpm, extracted at a temperature of 25 °C for 8 min, left to stand for stratification for 36 s, and then separated from the water phase to obtain an organic phase containing calcium ions.
[0038] The components of the nickel-cobalt-manganese leaching solution after removing iron and aluminum are shown in Table 2, and the detection results of calcium content in the raffinate are shown in Table 2.
Embodiment 3
[0040] A method for extracting calcium without saponification, comprising the steps:
[0041] (1) adjusting the pH of the nickel-cobalt-manganese leaching solution to be 5.5;
[0042] (2) 15% P204 (bis(2-ethylhexyl) phosphate), 10% TBP (tributyl phosphate) and 75% sulfonated kerosene are prepared into organic phase by mass percentage, take 100 mL of organic phase and 100 mL of The nickel-cobalt-manganese leaching solution was placed in a separatory funnel, oscillated at a rotational speed of 300 rpm, extracted at a temperature of 25 °C for 8 min, left to stand for stratification for 38 s, and then separated from the water phase to obtain an organic phase containing calcium ions.
[0043] The components of the nickel-cobalt-manganese leaching solution after removing iron and aluminum are shown in Table 2, and the detection results of calcium content in the raffinate are shown in Table 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| extraction efficiency | aaaaa | aaaaa |
| extraction efficiency | aaaaa | aaaaa |
| extraction efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com