Chondroitinase AC mutant, encoding gene, vector, engineering bacterium and preparation method of chondroitinase AC mutant
A technology for chondroitinase sulfate and encoding gene, applied in the field of genetic engineering, can solve the problems of low efficiency and limited catalytic activity, and achieve the effects of expanding the binding pocket, improving the activity, and reducing the binding steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Chondroitinase AC mutant protein
[0029] This embodiment provides an AC mutant of chondroitinase, which has the amino acid sequence shown in SEQ ID NO:2.
[0030] This embodiment also provides a gene encoding the above-mentioned chondroitinase AC mutant, which has the nucleotide sequence shown in SEQ ID NO:3.
Embodiment 2
[0031] Example 2 Construction of wild-type chondroitinase AC recombinant vector
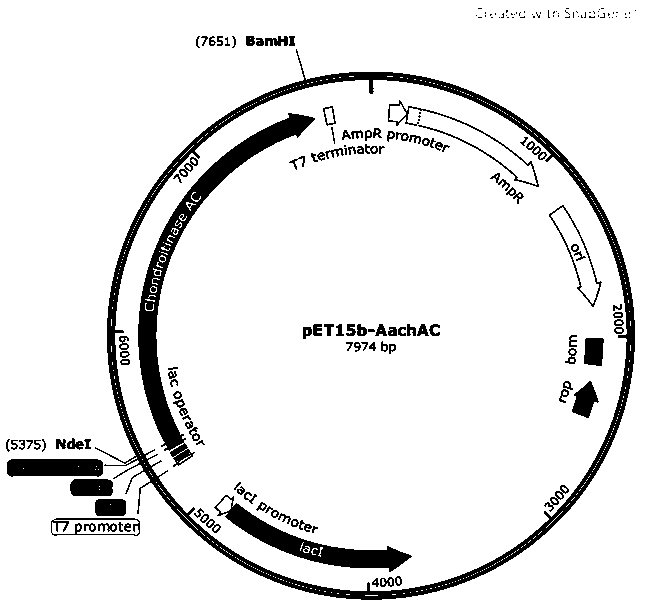
[0032] Using the wild-type chondroitinase AC shown in SEQ ID NO:1 as a template, the original primers were designed for PCR amplification, and NdeI and BamHI restriction enzyme sites were introduced, and then respectively connected to the plasmid vector for gene recombination construction to obtain wild-type chondroitinase AC. Type chondroitinase AC recombinant vector, and its N-terminus is linked to a his tag for subsequent isolation and purification of the chondroitinase AC mutant. The plasmid vector can be pET15b vector, PMAL-C2X or pGEX-4T1. In this example, the plasmid vector is the pET15b vector, and the wild-type chondroitinase AC recombinant vector is named pET-15b-AachAC. For a schematic diagram, see figure 1 .
[0033] Wherein, the original upstream primer F: 5 ’ -gggaaacatatgGAAGCTGAGCCAGGTGCAGCTG-3 ’ (Among them, the lowercase letters are mainly used for modification, and the upp...
Embodiment 3
[0035] Example 3 Construction of chondroitinase AC mutant and its recombinant vector
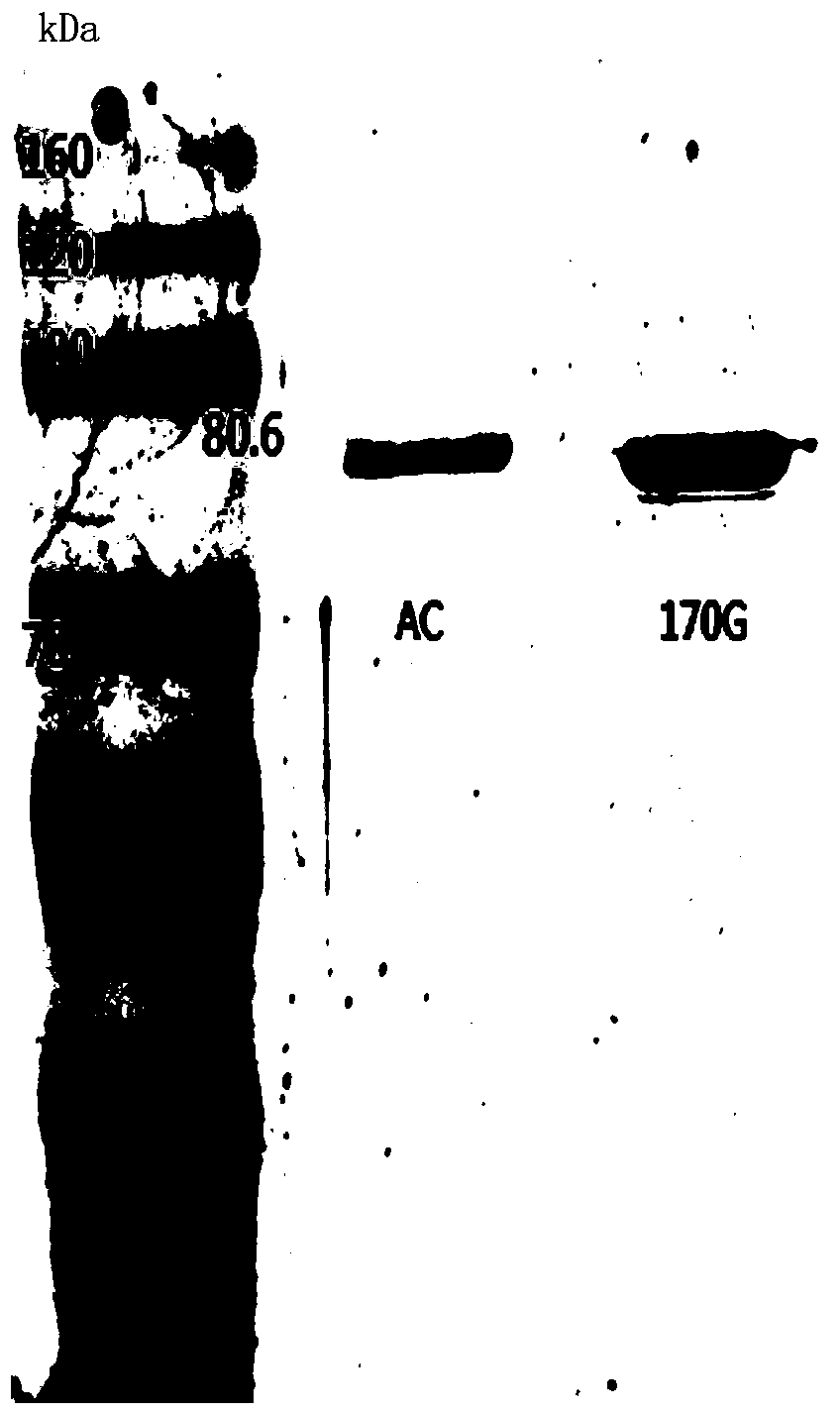
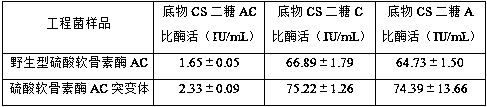
[0036] Using the wild-type chondroitinase AC recombinant vector pET-15b-AachAC provided in Example 2 as a template, the 170th DNA sequence in the amino acid sequence shown in SEQ ID NO: 1 is designed into the upstream primer, so that The 170th position is mutated to glycine, and then reverse PCR amplification is performed to obtain the PCR product. The PCR product does not need to be recovered by gel, and is digested with FD DpnI endonuclease at 37°C for 1 h to obtain the recombinant chondroitinase AC mutant. The vector was named pET-15b-AachAC(170G).
[0037] Wherein, the upstream primer F-170G: 5 ’ -GATCCGTGGCTGCAAGGTCCGCCTAAACGT-3 ’ (SEQ ID NO: 4);
[0038] The downstream primer R-170G: 5 ’ -TTGCAGCCACGGATCTGGGACGAAG-3 ’ (SEQ ID NO: 5).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com