Attenuated recombinant bacterium, preparation method thereof, application, and tumor targeted medicine
A technology of recombinant engineering bacteria and tumor targeting, which is applied in the field of tumor targeting drugs, attenuated recombinant engineering bacteria and its preparation, can solve the lack of early diagnosis and treatment measures for pancreatic cancer, the survival rate of pancreatic cancer patients is less than 8%, and the Cancer has no significant therapeutic effect and other problems, and achieves good tumor suppression effect, inhibition of pancreatic cancer growth, and high tumor targeting specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] An attenuated Salmonella typhimurium strain (ΔppGpp) of the present invention is synthesized by two synthetases, PSI and PSII, respectively encoded by relA and spotT genes, and inhibits the synthesis of ppGpp by constructing double deletion mutants of the relA and spotT genes.
[0040] Its concrete preparation method is:
[0041] (1) Design two pairs of primers for the relA and spotT gene sequences, each primer contains at least 40 bp complementary to the target gene, and 20 bp complementary to the template plasmid.
[0042] The relA forward primer sequence is:
[0043] 5'-gtggatcgcaagcctgggaatttccagccagcagtcgtgtgagcgcttaggtgtaggctggagctgcttc-3' (SEQ ID NO. 4);
[0044] The relA reverse primer sequence is:
[0045] 5'-gtgcagtcgccgtgcatcaatcacatccggcacctggttcagcttaccgaattccggggatccgtcgacc-3' (SEQ ID NO. 5);
[0046] The sequence of the spotT forward primer is:
[0047] 5'-ttaagcgtcttcggcaggcgtatctcgttgcacgtgacgctcacgagggctgtaggctggagctgcttc-3' (SEQ ID NO. 6);
[0048]...
Embodiment 2
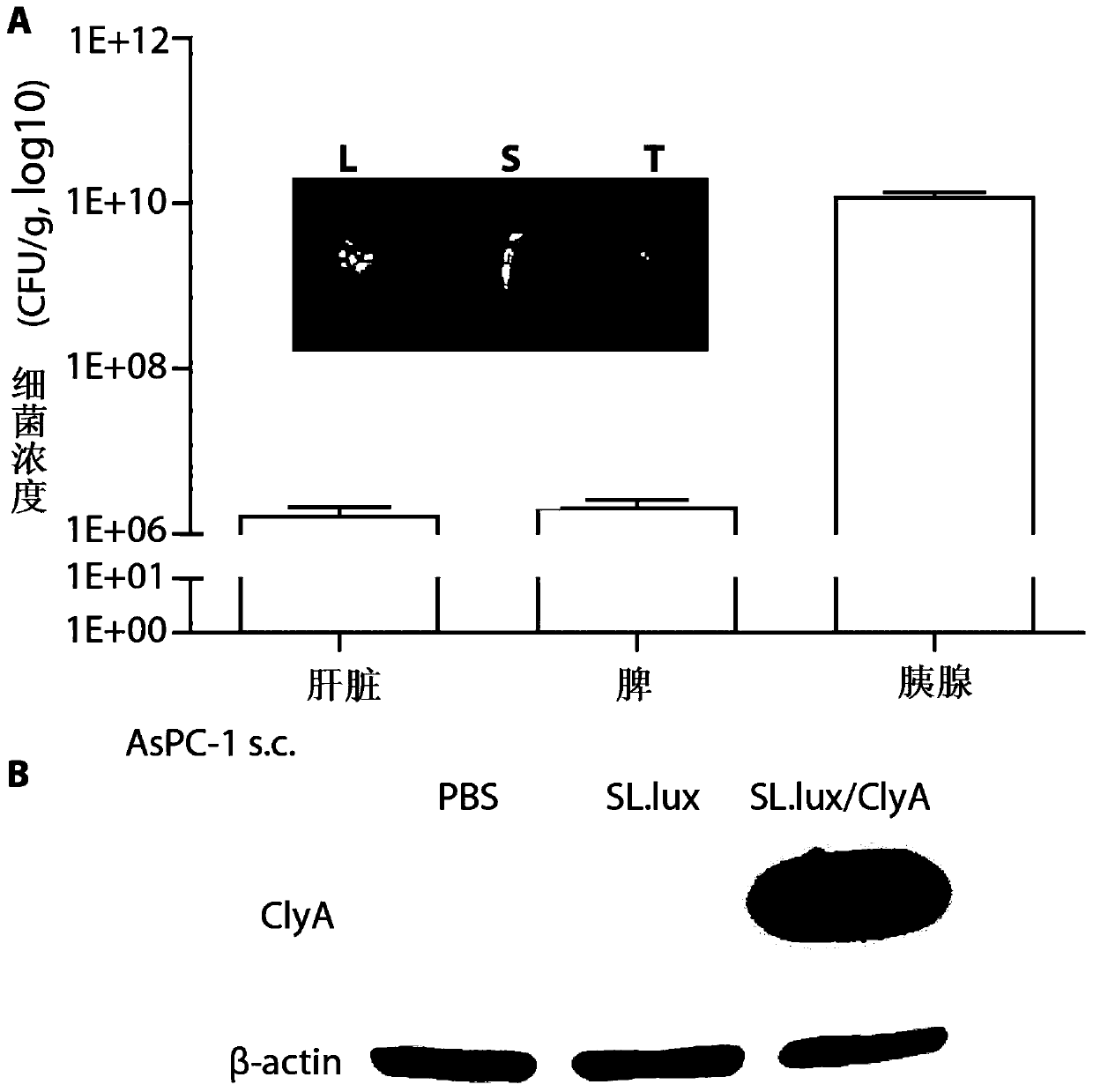
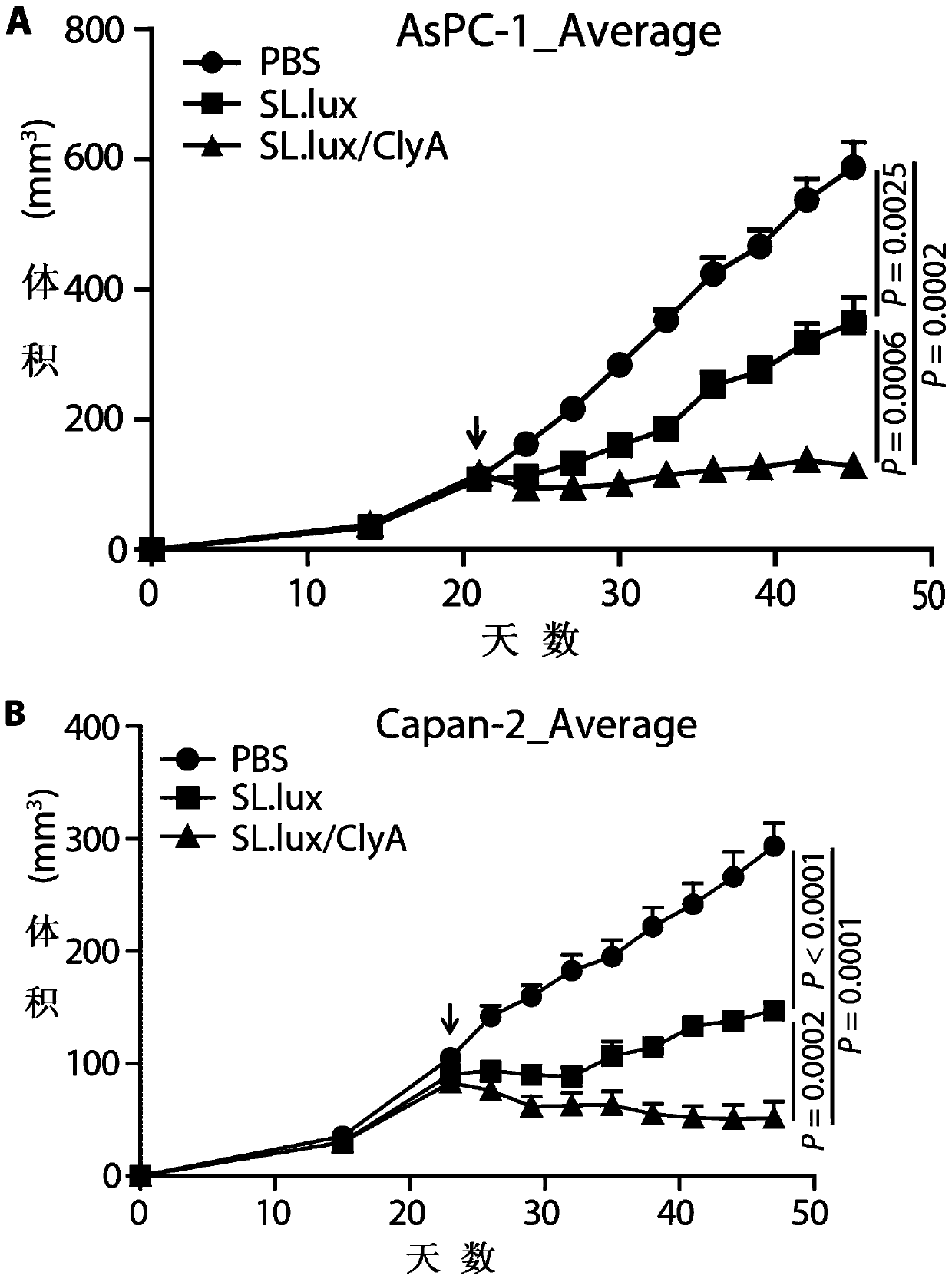
[0067] An attenuated recombinant engineering bacterium (SL.lux / ClyA) carrying a plasmid encoding ClyA, the preparation method of which comprises the following steps:
[0068] (1) Prepare the recombinant plasmid encoding ClyA:
[0069] 1.1. Using human Salmonella typhi (Salmonella typhi) genomic DNA as a template, the ClyA gene fragment was amplified by PCR. The specific primer sequences are as follows:
[0070] ClyA gene (SEQ ID NO.1):
[0071] atgaccggaatatttgcagaacaaactgtagaggtagttaaaagcgcgatcgaaaccgcagatggggcattagatctttataacaaatacctcgaccaggtcatcccctggaagacctttgatgaaaccataaaagagttaagccgttttaaacaggagtactcgcaggaagcttctgttttagttggtgatattaaagttttgcttatggacagccaggacaagtattttgaagcgacacaaactgtttatgaatggtgtggtgtcgtgacgcaattactctcagcgtatattttactatttgatgaatataatgagaaaaaagcatcagcccagaaagacattctcattaggatattagatgatggtgtcaagaaactgaatgaagcgcaaaaatctctcctgacaagttcacaaagtttcaacaacgcttccggaaaactgctggcattagatagccagttaactaatgatttttcggaaaaaagtagttatttccagtcacaggtggatagaattcgtaaggaagcttatgccggtg...
Embodiment 3
[0082] In vitro activity identification of attenuated recombinant engineering bacteria expressing ClyA:
[0083] 1. Cultivation of recombinant engineered bacteria:
[0084] Experimental group: the attenuated recombinant engineered bacteria (SL.lux / ClyA) carrying the plasmid encoding ClyA of Example 2;
[0085] Control group: the attenuated Salmonella typhimurium (SL.lux) that does not carry the plasmid encoding ClyA in Example 1.
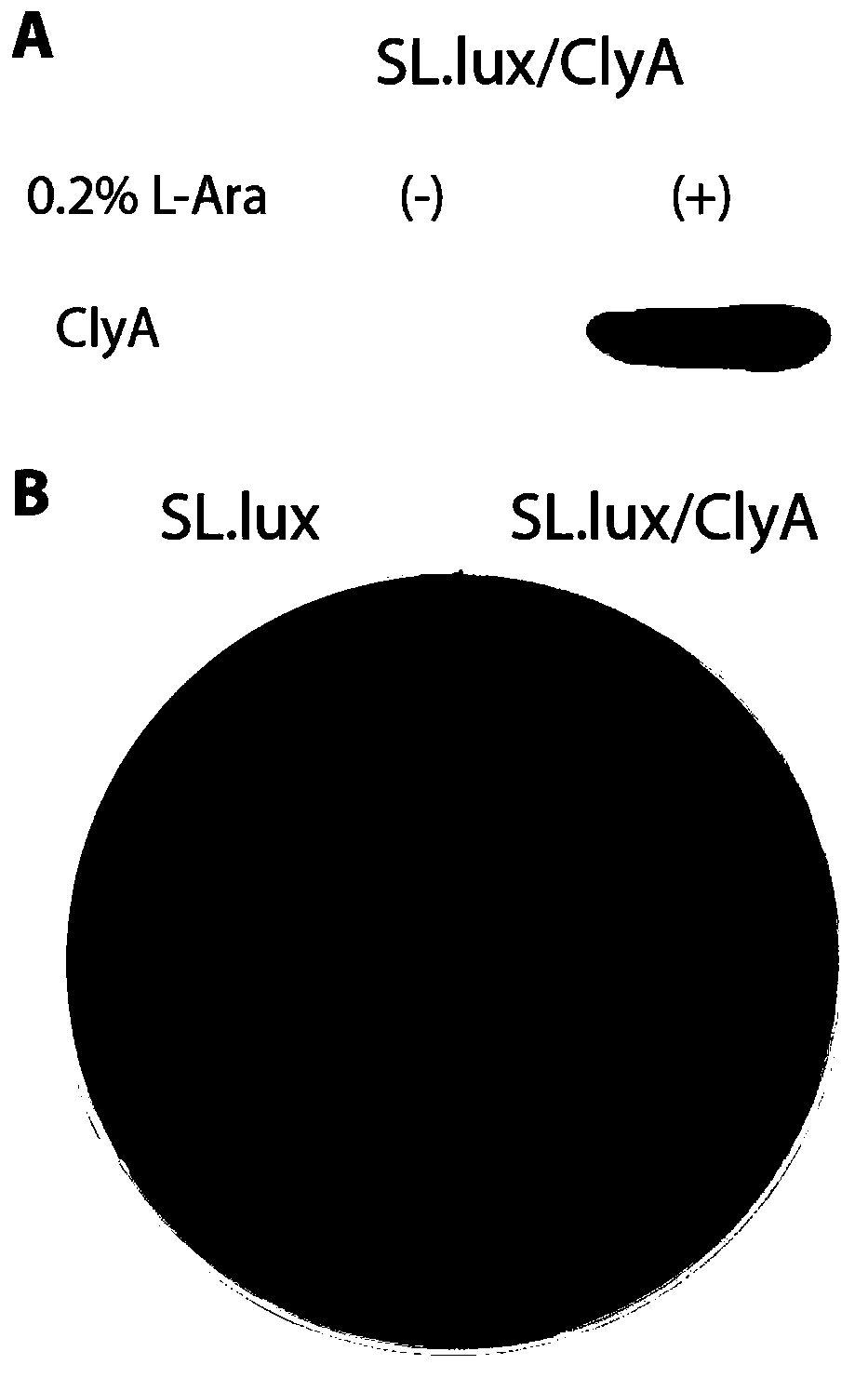
[0086] 2. Hemolysis test:
[0087] 2.1. Evenly smear 100 μl of 40% L-arabinose on a fresh blood plate with a diameter of 10 cm, and inoculate the attenuated recombinant engineered bacteria (SL. Plasmids of attenuated Salmonella typhimurium (SL.lux).
[0088] 2.2. Incubate overnight at 37°C to observe the hemolysis of the plate to confirm the expression and activity of ClyA.
[0089] 3. Detection of ClyA expression by Western blot:
[0090]3.1. Take single colonies of SL.lux / ClyA and SL.lux from the plates in step 2.2, and culture them overnight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com