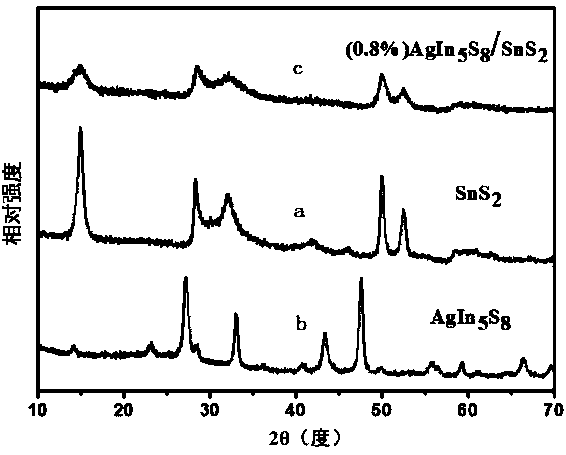
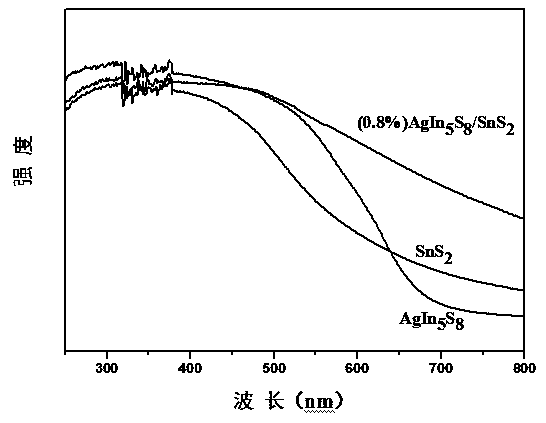
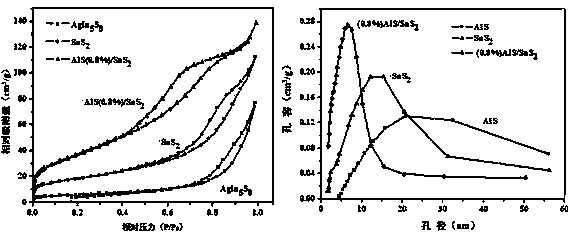
Preparation method of AgIn5S8/SnS2 solid solution catalyst with adsorption-photocatalysis synergistic effect
A synergistic effect and catalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve problems such as poor utilization rate, achieve high adsorption capacity, strong light absorption, and strong photocatalysis The effect of degradability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) 3.5 g SnCl 4 ·5H 2 O was dispersed in 60 mL of deionized water under continuous magnetic stirring, and stirred at room temperature for 30 minutes to obtain a pale yellow mixed solution;
[0030] (2) Slowly add 2.254 g (30 mmol) of thioacetamide and 1.921 g (10 mmol) of citric acid into the above mixed solution in sequence, and stir magnetically for 2 h at room temperature;
[0031] (3) Transfer the fully reacted solution to a reaction kettle, and conduct a hydrothermal reaction at 150 °C for 12 hours. After the reaction is completed, it is naturally cooled to room temperature, vacuum filtered through a 0.22 m filter membrane, and then deionized water and anhydrous Washed several times with ethanol and dried at 60°C for 24 h. You can get AgIn 5 S 8 / SnS 2 solid solution catalyst.
Embodiment 2
[0033] (1) 0.05 g AgNO 3 , 4.5 g InCl 3 ·5H 2 O and 0.45 g of thioacetamide were dispersed in 60 mL of deionized water under continuous magnetic stirring, and stirred at room temperature for 2 h to obtain a pale yellow mixed solution;
[0034] (2) Transfer the fully reacted solution to a reaction kettle, and conduct a hydrothermal reaction at a temperature of 150 °C for 12 hours. After the reaction is completed, naturally cool to room temperature, vacuum filter through a 0.22 m filter membrane, and then use deionized water and anhydrous Washed several times with ethanol and dried at 60°C for 24 h. You can get AgIn 5 S 8 / SnS 2 solid solution catalyst.
Embodiment 3
[0036] (1) 3.5 g SnCl 4 ·5H 2O, 0.01 g AgNO 3 and 0.15 g InCl 3 ·5H 2 O was dispersed in 60 mL of deionized water under continuous magnetic stirring, and stirred at room temperature for 30 minutes to obtain a pale yellow mixed solution;
[0037] (2) Slowly add 2.254 g (30 mmol) of thioacetamide and 1.921 g (10 mmol) of citric acid into the above mixed solution in sequence, and stir magnetically for 2 h at room temperature;
[0038] (3) Transfer the fully reacted solution to a reaction kettle, and conduct a hydrothermal reaction at 150 °C for 12 hours. After the reaction is completed, it is naturally cooled to room temperature, vacuum filtered through a 0.22 m filter membrane, and then deionized water and anhydrous Washed several times with ethanol and dried at 60°C for 24 h. You can get AgIn 5 S 8 / SnS 2 solid solution catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com