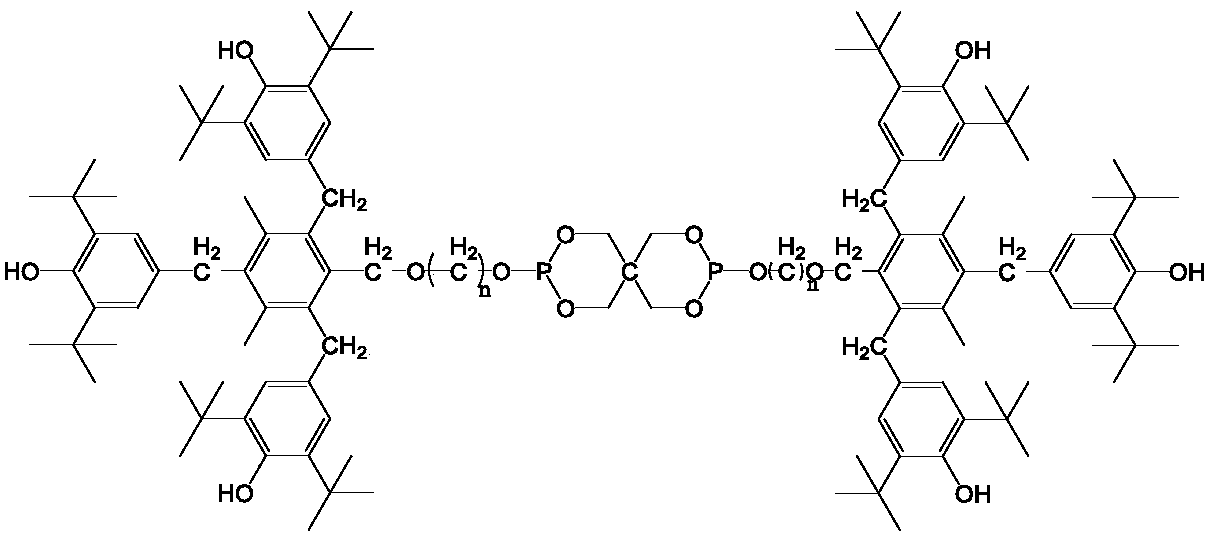
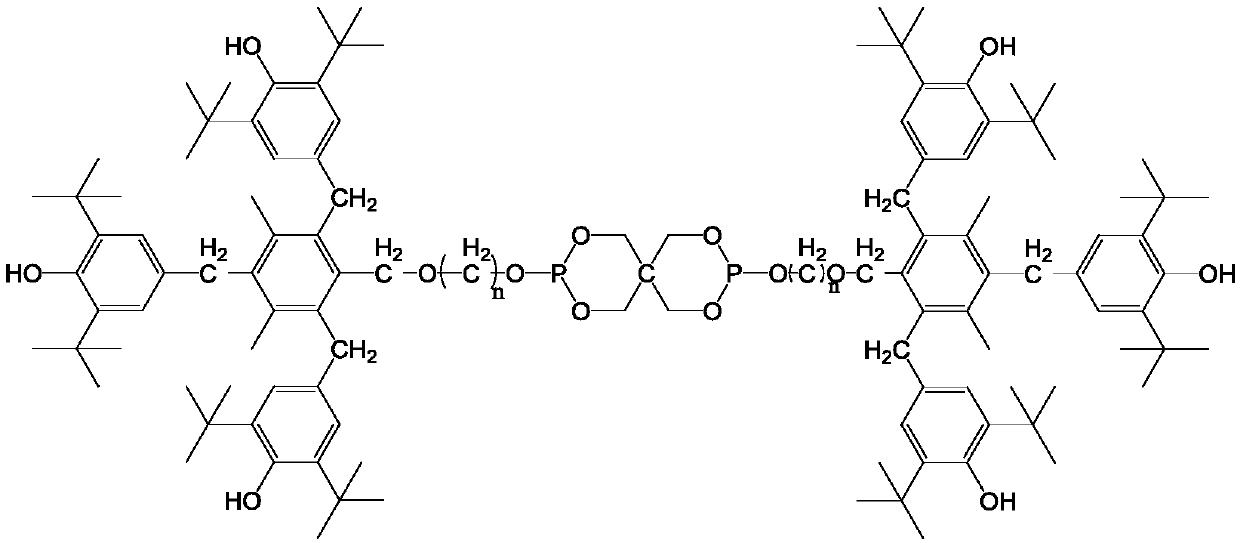
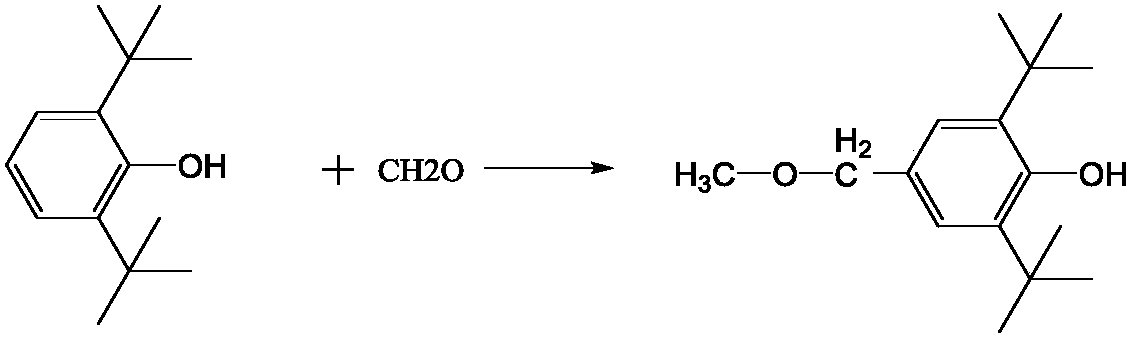
Compound containing hindered phenol and pentaerythritol structure, and synthesis method thereof, and applications as antioxidant
A technology of pentaerythritol and synthesis method, applied in the field of multifunctional compounds and synthesis thereof, can solve the problems of affecting health, precipitation of antioxidants, harm to human health and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Add 293.5g of 2,6-di-tert-butylphenol, 68.4g of paraformaldehyde, 0.7L of methanol, 2.8g of piperazine and 7.1g of ethylenediamine into a 1.5L reactor, and raise the temperature of the reaction solution to 135°C. Heating was continued under stirring for 3 h and then the heating was stopped. After the reaction solution returned to room temperature, the reaction solution was filtered. The solid product was washed three times with 0.45 L of methanol each time, and the solid was dried to obtain 299.2 g of white product 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, with a yield of 84%.
[0090]In a 2.5L three-neck round bottom flask, add 1L of dichloromethane, add 38.9g of mesitylene and 299.2g of 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, and control the temperature of the reaction solution under an ice-salt bath At 0°C, under slow stirring, 104.7 g of concentrated sulfuric acid with a concentration of 0.84% was added dropwise to the reaction solution. The dropwis...
Embodiment 2
[0097] Add 293.5g of 2,6-di-tert-butylphenol, 68.4g of paraformaldehyde, 0.7L of methanol, 2.8g of piperazine and 7.1g of ethylenediamine into a 1.5L reactor, and raise the temperature of the reaction solution to 135°C. Heating was continued under stirring for 3 h and then the heating was stopped. After the reaction solution returned to room temperature, the reaction solution was filtered. The solid product was washed three times with 0.45 L of methanol each time, and the solid was dried to obtain 299.2 g of white product 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, with a yield of 84%.
[0098] In a 2.5L three-neck round bottom flask, add 1L of dichloromethane, add 38.9g of mesitylene and 299.2g of 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, and control the temperature of the reaction solution under an ice-salt bath At 0°C, under slow stirring, 104.7 g of concentrated sulfuric acid with a concentration of 0.84% was added dropwise to the reaction solution. The dropwi...
Embodiment 3
[0105] Add 293.5g of 2,6-di-tert-butylphenol, 68.4g of paraformaldehyde, 0.7L of methanol, 2.8g of piperazine and 7.1g of ethylenediamine into a 1.5L reactor, and raise the temperature of the reaction solution to 135°C. Heating was continued under stirring for 3 h and then the heating was stopped. After the reaction solution returned to room temperature, the reaction solution was filtered. The solid product was washed three times with 0.45 L of methanol each time, and the solid was dried to obtain 299.2 g of white product 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, with a yield of 84%.
[0106]In a 2.5L three-neck round bottom flask, add 1L of dichloromethane, add 38.9g of mesitylene and 299.2g of 3,5-di-tert-butyl-4-hydroxybenzyl methyl ether, and control the temperature of the reaction solution under an ice-salt bath At 0°C, under slow stirring, 104.7 g of concentrated sulfuric acid with a concentration of 0.84% was added dropwise to the reaction solution. The dropwis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com