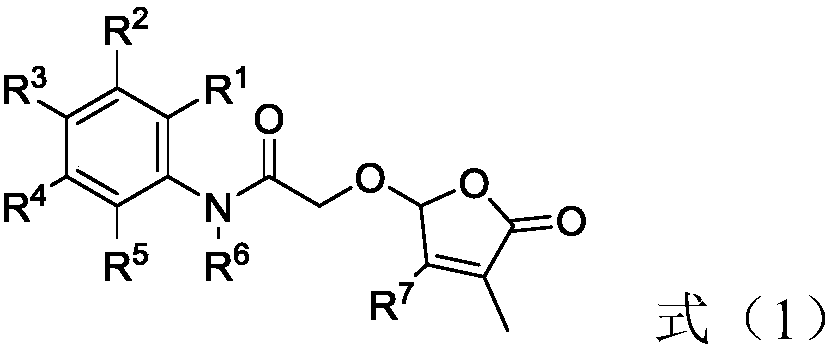
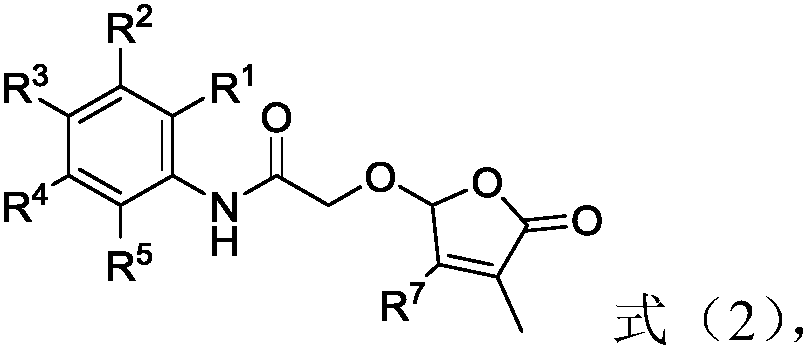
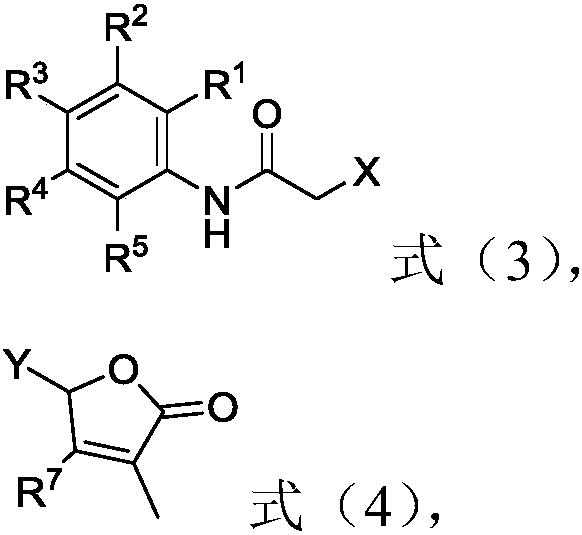
2-butenolide acetamide compound, and preparation method and application thereof
A technology of ester acetamide and butenoic acid, applied in the field of pesticide chemistry, can solve the problems of inapplicability in the field and poor stability, and achieve the effect of promoting germination and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0093] Synthesis of compound 1
[0094] Potassium carbonate (1.9 g), 5-hydroxy-3-methylfuran-2(5H)-one (0.64 g) were added into the reaction flask, and acetone (50 mL) was added under stirring. After reacting at room temperature for 15 minutes, 2-bromo-N-phenylacetamide (1.2 g) was added, and the stirring reaction was continued for 10 hours. After the reaction was completed. Diatomaceous earth assisted suction filtration to remove insoluble matter, and the acetone in the solution was removed under reduced pressure, and the obtained residue was subjected to column chromatography to obtain 0.95 g of colorless oily compound 1, and its yield (calculated by weight, the same below) was 68% , the NMR data are 1 H NMR (400MHz, CDCl 3 )δ8.07(s,1H),7.54(d,J=7.6Hz,2H),7.34(t,J=8.0Hz,3H),7.15(t,J=7.6Hz,1H),6.95–6.90( m, 1H), 5.94 (s, 1H), 4.34 (dd, J=35.6, 14.8Hz, 2H), 2.17 (s, 3H).
preparation example 2
[0096] Synthesis of compound 1
[0097] Cesium carbonate (4.6g) and 2-hydroxy-N-phenylacetamide (0.8g) were added to the reaction flask, and N,N-dimethylformamide (50mL) was added under stirring. After reacting at room temperature for 15 minutes, 1.03 g of 5-bromo-3-methylfuran-2(5H)-one was added, and the stirring reaction was continued for 10 hours. After the reaction was completed. Add 100 mL of ice water and 100 mL of ethyl acetate to the system, and stir vigorously for 10 min. The organic layer was separated, and the aqueous layer was extracted once with 50 mL of ethyl acetate, and the organic layers were combined. The organic layer was extracted once with 50 mL of water and 50 mL of saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure. The resulting residue was subjected to column chromatography to obtain 0.46 g of a colorless oily compound 1 with a yield of 46 %, NMR data is 1 H NMR (400MHz, CDCl 3 )δ8.07(s,1H),7.5...
preparation example 3
[0099] Synthesis of compound 1
[0100] Potassium carbonate (1.2g), N,N-diisopropylethylamine (1.1g), 5-hydroxy-3-methylfuran-2(5H)-one (0.64g) were added to the reaction flask, stirred Acetonitrile (50 mL) was added at the same time. After reacting at room temperature for 15 minutes, 2-bromo-N-phenylacetamide (1.2 g) was added, and the stirring reaction was continued for 10 hours. After the reaction was completed. Diatomaceous earth assisted suction filtration to remove insoluble matter, and the acetonitrile in the solution was removed under reduced pressure, and the resulting residue was subjected to column chromatography to obtain 1.1 g of colorless oily compound 1, and the yield was 78%, and the NMR data was 1 H NMR (400MHz, CDCl 3 )δ8.07(s,1H),7.54(d,J=7.6Hz,2H),7.34(t,J=8.0Hz,3H),7.15(t,J=7.6Hz,1H),6.95–6.90( m, 1H), 5.94 (s, 1H), 4.34 (dd, J=35.6, 14.8Hz, 2H), 2.17 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com